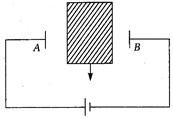
question_answer 1) If E = energy, G = gravitational constant, \[I=\] impulse and M = mass, then dimensions of \[\frac{GI{{M}^{2}}}{{{E}^{2}}}\] are same as that of
A)
time
done
clear
B)
mass
done
clear
C)
length
done
clear
D)
force
done
clear
View Answer play_arrow
question_answer 2) A point initially at rest moves along x-axis. Its acceleration varies with time as\[a=(6\,t+5)\,m/{{s}^{2}}\]. If it starts from origin, the distance covered in 2 s is
A)
20 m
done
clear
B)
18 m
done
clear
C)
16 m
done
clear
D)
25 m
done
clear
View Answer play_arrow
question_answer 3)
Three blocks of masses 2 kg, 3 kg and 5 kg are connected to each other with light st ring and are then placed on a friction less surface as shown in the figure. The system is pulled by a force F = 10 N, then tension \[{{T}_{1}}=\]
A)
1 N
done
clear
B)
5 N
done
clear
C)
8N
done
clear
D)
ION
done
clear
View Answer play_arrow
question_answer 4) A solid sphere rolls down two different inclined planes of same height, but of different inclinations. In both cases
A)
speed and time of descent will be same
done
clear
B)
speed will be same, but time of descent will be different
done
clear
C)
speed will be different, but time of descent will be same
done
clear
D)
speed and time of descent both are different
done
clear
View Answer play_arrow
question_answer 5) The angular amplitude of a simple pendulum is\[{{\theta }_{0}}\]. The maximum tension in its string will be
A)
\[mg\,(1-{{\theta }_{0}})\]
done
clear
B)
\[mg\,(1+{{\theta }_{0}})\]
done
clear
C)
\[mg\,(1-\theta _{0}^{2})\]
done
clear
D)
\[mg\,(1+\theta _{0}^{2})\]
done
clear
View Answer play_arrow
question_answer 6) An engine pumps up 100 kg of water through a height of 10 m in 5s. Given that the efficiency of engine is 60%. If \[g=10\,m{{s}^{-2}}\], the power of the engine is
A)
3.3 kW
done
clear
B)
0.33 kW
done
clear
C)
0.033 kW
done
clear
D)
33 kW
done
clear
View Answer play_arrow
question_answer 7) At what speed, the velocity head of water is equal to pressure head of 40 cm of Hg?
A)
10.3 m/s
done
clear
B)
2.8 m/s
done
clear
C)
5.6 m/s
done
clear
D)
8.4 m/s
done
clear
View Answer play_arrow
question_answer 8) If the electric flux entering and leaving an enclosed surface respectively are \[{{\phi }_{1}}\] and \[{{\phi }_{2}}\], the electric charge inside the surface will be
A)
\[\frac{{{\phi }_{2}}-{{\phi }_{1}}}{{{\varepsilon }_{0}}}\]
done
clear
B)
\[\frac{{{\phi }_{1}}+{{\phi }_{2}}}{{{\varepsilon }_{0}}}\]
done
clear
C)
\[\frac{{{\phi }_{1}}-{{\phi }_{2}}}{{{\varepsilon }_{0}}}\]
done
clear
D)
\[{{\varepsilon }_{0}}\,({{\phi }_{1}}+{{\phi }_{2}})\]
done
clear
View Answer play_arrow
question_answer 9) In the propagation of light waves, the angle between the direction of vibration and plane of polarisation is
A)
\[{{0}^{o}}\]
done
clear
B)
\[{{90}^{o}}\]
done
clear
C)
\[{{45}^{o}}\]
done
clear
D)
\[{{80}^{o}}\]
done
clear
View Answer play_arrow
question_answer 10) A light emitting diode (LED) has a voltage drop of 2 V across it and passes a current of 10 mA. When it operates with a 6 V battery through a limiting resistor R, the value of R is
A)
\[40\,\,k\Omega \]
done
clear
B)
\[4\,\,k\Omega \]
done
clear
C)
\[200\,\,k\Omega \]
done
clear
D)
\[400\,\,k\Omega \]
done
clear
View Answer play_arrow
question_answer 11) The minimum potential difference between the base and emitter required to switch a silicon transistor ON is approximately
A)
IV
done
clear
B)
3V
done
clear
C)
5V
done
clear
D)
4.2V
done
clear
View Answer play_arrow
question_answer 12) Two stones are projected with the same speed but making different angles with the horizontal. Their horizontal ranges are equal. The angle of projection of one is \[\pi /3\] and the maximum height reached by it is 102 m. Then the maximum height reached by the other in metre is
A)
336
done
clear
B)
224
done
clear
C)
56
done
clear
D)
34
done
clear
View Answer play_arrow
question_answer 13) Which one of the following is a possible nuclear reaction?
A)
\[_{5}^{10}B+_{2}^{4}He\xrightarrow{{}}_{7}^{13}N+_{1}^{1}H\]
done
clear
B)
\[_{11}^{23}Na+_{1}^{1}H\xrightarrow{{}}_{10}^{20}Ne+_{2}^{4}He\]
done
clear
C)
\[_{93}^{293}Np\xrightarrow{{}}_{94}^{293}Pu+{{\beta }^{-}}+\overline{v}\]
done
clear
D)
\[_{7}^{11}N+_{1}^{1}H\xrightarrow{{}}_{6}^{12}C+{{\beta }^{-}}+v\]
done
clear
View Answer play_arrow
question_answer 14)
Two circular discs A and B with equal radii are blackened. They are heated to same temperature and are cooled under identical
A)
A and B have same specific heats
done
clear
B)
Specific heat of A is less
done
clear
C)
Specific heat of B is less
done
clear
D)
Nothing can be said
done
clear
View Answer play_arrow
question_answer 15) During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio \[{{C}_{p}}/{{C}_{V}}\]for the gas is
A)
4/3
done
clear
B)
2
done
clear
C)
5/3
done
clear
D)
3/2
done
clear
View Answer play_arrow
question_answer 16) The sum of two vectors \[\vec{A}\] and \[\vec{B}\] is at right angles to their difference. Then
A)
A = B
done
clear
B)
A = 2B
done
clear
C)
B = 2A
done
clear
D)
\[\vec{A}\] and \[\vec{B}\] have the same direction
done
clear
View Answer play_arrow
question_answer 17) A ball is thrown up at an angle with the horizontal. Then the total change of momentum by the instant it returns to ground is
A)
acceleration due to gravity x total time of flight
done
clear
B)
weight of the ball x half the time of flight
done
clear
C)
weight of the ball x total time of flight
done
clear
D)
weight of the ball x horizontal range
done
clear
View Answer play_arrow
question_answer 18) When a spring is stretched by a distance \[x\], it exerts a force, given by \[F=(-5x-16{{x}^{3}})N\] The work done, when the spring is stretched from 0.1 m to 0.2 m is
A)
\[8.7\times {{10}^{-2}}J\]
done
clear
B)
\[12.2\times {{10}^{-2}}J\]
done
clear
C)
\[8.7\times {{10}^{-1}}J\]
done
clear
D)
\[12.2\times {{10}^{-1}}J\]
done
clear
View Answer play_arrow
question_answer 19) Time period of a simple pendulum of length I is \[{{T}_{1}}\] and time period of a uniform rod of the same length \[l\] pivoted about one end and oscillating in a vertical plane is \[{{T}_{2}}\]. Amplitude of oscillations in both the cases is small. Then\[{{T}_{1}}/{{T}_{2}}\] is
A)
\[\frac{1}{\sqrt{3}}\]
done
clear
B)
1
done
clear
C)
\[\sqrt{\frac{4}{3}}\]
done
clear
D)
\[\sqrt{\frac{3}{2}}\]
done
clear
View Answer play_arrow
question_answer 20)
An insulator plate is passed between the plates of a capacitor. Then current
A)
first flows from A to B and then from B to A
done
clear
B)
first flows from B to A and then from A to B
done
clear
C)
always flows from B to A
done
clear
D)
always flows from A to B
done
clear
View Answer play_arrow
question_answer 21) Two parallel large thin metal sheets have equal surface charge densities \[(\sigma =(26.4\times {{10}^{-12}}C/{{m}^{2}})\] of opposite signs. The electric field between these sheets is
A)
1.5 N/C
done
clear
B)
\[1.5\times {{10}^{-10}}N/C\]
done
clear
C)
3 N/C
done
clear
D)
\[3\times {{10}^{-10}}N/C\]
done
clear
View Answer play_arrow
question_answer 22) The electric current passes through a metallic wire produces heat because of
A)
collisions of conduction electrons with each other
done
clear
B)
collisions of the atoms of the metal with each other
done
clear
C)
the energy released in the ionization of the atoms of the metal
done
clear
D)
collisions of the conduction electrons with the atoms of the metallic wire
done
clear
View Answer play_arrow
question_answer 23) Two concentric circular coils of ten turns each are situated in the same plane. Their radii are 20 cm and 40 cm and they carry respectively 0.2 A and 0.3 A currents in opposite direction. The magnetic field in tesla at the centre is
A)
\[35{{\mu }_{0}}/4\]
done
clear
B)
\[{{\mu }_{0}}/80\]
done
clear
C)
\[7{{\mu }_{0}}/80\]
done
clear
D)
\[5{{\mu }_{0}}/4\]
done
clear
View Answer play_arrow
question_answer 24) Electromagnetic waves with frequencies greater than the critical frequency of ionosphere cannot be used for communication using sky wave propagation, because
A)
the refractive index of the ionosphere becomes very high for \[f>{{f}_{c}}\]
done
clear
B)
the refractive index of the ionosphere becomes very low for \[f>{{f}_{c}}\]
done
clear
C)
the refractive index of the ionosphere becomes very high for \[f>{{f}_{c}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 25) In a choke coil, the reactance \[{{X}_{L}}\] and resistance R are such that
A)
\[{{X}_{L}}=R\]
done
clear
B)
\[{{X}_{L}}>>R\]
done
clear
C)
\[{{X}_{L}}<<R\]
done
clear
D)
\[{{X}_{L}}=\infty \]
done
clear
View Answer play_arrow
question_answer 26) Reverberation time does not depend upon
A)
temperature
done
clear
B)
volume of room
done
clear
C)
size of window
done
clear
D)
carpet and curtain
done
clear
View Answer play_arrow
question_answer 27) In a potentiometer, the null point is received at 7th wire. If now we have to change the null point at 9th wire, what should we do?
A)
Attach resistance in series with battery
done
clear
B)
Increase resistance in main circuit
done
clear
C)
Decrease resistance in main circuit
done
clear
D)
Decrease applied emf
done
clear
View Answer play_arrow
question_answer 28) If average velocity becomes 4 times then what will be the effect on rms velocity at that temperature?
A)
1.4 times
done
clear
B)
4 times
done
clear
C)
2 times
done
clear
D)
\[\frac{1}{4}\]times
done
clear
View Answer play_arrow
question_answer 29) What is the Q-value of the reaction \[p{{+}^{7}}Li{{\xrightarrow{{}}}^{4}}He{{+}^{4}}He\] The atomic masses of \[^{1}H,{{\,}^{4}}He\] and \[^{7}Li\] are 1.007825 u, 4.002603 u and 7.016004 u respectively
A)
17.35 MeV
done
clear
B)
18.06 MeV
done
clear
C)
177.35 MeV
done
clear
D)
170.35 MeV
done
clear
View Answer play_arrow
question_answer 30) A Carnots engine has an efficiency of 50% at sink temperature \[{{50}^{o}}C\]. Calculate the temperature of source.
A)
\[{{133}^{o}}C\]
done
clear
B)
\[{{143}^{o}}C\]
done
clear
C)
\[{{100}^{o}}C\]
done
clear
D)
\[{{373}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 31) \[4\,\,{{m}^{3}}\] of water is to be pumped to a height of 20 m and forced into a reservoir at a pressure of \[2\times {{10}^{5}}N/{{m}^{2}}\]. The work done by the motor is (external pressure \[={{10}^{5}}N/{{m}^{2}}\])
A)
\[8\times {{10}^{5}}J\]
done
clear
B)
\[16\times {{10}^{5}}J\]
done
clear
C)
\[12\times {{10}^{5}}J\]
done
clear
D)
\[32\times {{10}^{5}}J\]
done
clear
View Answer play_arrow
question_answer 32) Moment of inertia of ring about its diameter is Then, moment of inertia about an axis passing through centre perpendicular to its plane is
A)
\[2I\]
done
clear
B)
\[\frac{I}{2}\]
done
clear
C)
\[\frac{3}{2}I\]
done
clear
D)
\[I\]
done
clear
View Answer play_arrow
question_answer 33) When both the listener and source are moving towards each other, then which of the following is true regarding frequency and wavelength of wave observed by the observer?
A)
More frequency, less wavelength
done
clear
B)
More frequency, more wavelength
done
clear
C)
Less frequency, less wavelength
done
clear
D)
More frequency, constant wavelength
done
clear
View Answer play_arrow
question_answer 34) The coefficient of friction between the tyres and the road is 0.25. The maximum speed with which car can be driven round a curve of radius 40 m without skidding is (assume \[g=10\,\,m{{s}^{-2}}\])
A)
\[40\,\,m{{s}^{-1}}\]
done
clear
B)
\[20\,\,m{{s}^{-1}}\]
done
clear
C)
\[15\,\,m{{s}^{-1}}\]
done
clear
D)
\[10\,\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 35) A bomb of mass 3.0 kg explodes in air into two pieces of masses 2.0 kg and 1.0 kg. The smaller mass goes at a speed of 80 m/s. The total energy imparted to the two fragments is
A)
1.07 kJ
done
clear
B)
2.14 kJ
done
clear
C)
2.4 kJ
done
clear
D)
4.8 kJ
done
clear
View Answer play_arrow
question_answer 36) If distance between earth and sun become four times, then time period becomes
A)
4 times
done
clear
B)
8 times
done
clear
C)
1/4 times
done
clear
D)
1/8 times
done
clear
View Answer play_arrow
question_answer 37)
An air bubble is contained inside water. It behaves as a
A)
concave lens
done
clear
B)
convex lens
done
clear
C)
Neither convex nor concave
done
clear
D)
Cannot say
done
clear
View Answer play_arrow
question_answer 38) The power dissipated across resistance R which is connected across a battery of potential V is P. If resistance is doubled, then the power becomes
A)
1/2
done
clear
B)
2
done
clear
C)
1/4
done
clear
D)
4
done
clear
View Answer play_arrow
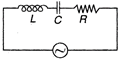
question_answer 39)
A 100 V, AC source of frequency 500 Hz is connected to an LCR circuit with L = 8.1 mH, C = 12.5 \[\mu F\], R = 10 \[\Omega \] all connected in series as shown in figure. What is the quality factor of circuit?
A)
2.02
done
clear
B)
2.5434
done
clear
C)
20.54
done
clear
D)
200.54
done
clear
View Answer play_arrow
question_answer 40) The inductance of a coil is \[L=10\,H\] and resistance \[R=5\,\Omega \]. If applied voltage of battery is 10 V and it switches off in 1 millisecond, find induced emf of inductor.
A)
\[2\times {{10}^{4}}V\]
done
clear
B)
\[1.2\times {{10}^{4}}V\]
done
clear
C)
\[2\times {{10}^{4}}V\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 41) A proton is moving in a uniform magnetic field B in a circular path of radius a in a direction perpendicular to z-axis along which field B exists. Calculate the angular momentum, if the radius is a charge on proton is e.
A)
\[\frac{Be}{{{a}^{2}}}\]
done
clear
B)
\[e{{B}^{2}}a\]
done
clear
C)
\[{{a}^{2}}eB\]
done
clear
D)
\[aeB\]
done
clear
View Answer play_arrow
question_answer 42) We wish to see inside an atom. Assuming the atom to have a diameter of 100 pm, this means that one must be able to resolve a width of say 10 pm. If an electron microscope is used, the minimum electron energy required is about
A)
1.5 keV
done
clear
B)
15 keV
done
clear
C)
150 keV
done
clear
D)
1.5 MeV
done
clear
View Answer play_arrow
question_answer 43) A body from height h is dropped. If the coefficient of restitution is e, then calculate the height achieved after one bounce.
A)
\[{{h}_{1}}={{e}^{2}}h\]
done
clear
B)
\[{{h}_{1}}={{e}^{4}}h\]
done
clear
C)
\[{{h}_{1}}=eh\]
done
clear
D)
\[{{h}_{1}}=\frac{h}{e}\]
done
clear
View Answer play_arrow
question_answer 44) A beam of light travelling along x-axis is described by the electric field \[{{E}_{y}}=(600\,\,V{{m}^{-1}})\,\sin \,\omega \,(t-x/c)\]then maximum magnetic force on a charge\[q=2e\], moving along y-axis with a speed of\[3.0\times {{10}^{7}}m{{s}^{-1}}\] is \[(e=1.6\times {{10}^{-19}}C)\]
A)
\[19.2\times {{10}^{-17}}N\]
done
clear
B)
\[1.92\times {{10}^{-17}}N\]
done
clear
C)
0.192 N
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 45) Equipotential surfaces associated with an electric field which is increasing in magnitude along the x-direction are
A)
planes parallel to \[Yz-plane\]
done
clear
B)
planes parallel to \[xy\] -plane
done
clear
C)
planes parallel to \[xz\]-plane
done
clear
D)
coaxial cylinders of increasing radii around the x-axis
done
clear
View Answer play_arrow
question_answer 46) If 200 MeV energy is released in the fission of a single nucleus of \[_{92}{{U}^{235}}\], how many fissions must occur per second to produce a power of 1 kW?
A)
\[3.12\times {{10}^{13}}\]
done
clear
B)
\[3.12\times {{10}^{3}}\]
done
clear
C)
\[3.1\times {{10}^{17}}\]
done
clear
D)
\[3.12\times {{10}^{19}}\]
done
clear
View Answer play_arrow
question_answer 47) Find ratio of acceleration due to gravity g at depth d and at height h, where\[d=2h\].
A)
1 : 1
done
clear
B)
1 : 2
done
clear
C)
2 : 1
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 48) A convex lens of focal length 40 cm is in contact with a concave lens of focal length 25 cm. The power of combination is
A)
-1.5D
done
clear
B)
-6.5D
done
clear
C)
+ 6.5D
done
clear
D)
4- 6.67 D
done
clear
View Answer play_arrow
question_answer 49) In a Youngs experiment, two coherent sources are placed 0.90 mm apart and the fringes are observed one metre away. If it produces the second dark fringe at a distance of 1 mm from the central fringe, the wavelength of monochromatic light used would be
A)
\[60\times {{10}^{-4}}cm\]
done
clear
B)
\[10\times {{10}^{-4}}cm\]
done
clear
C)
\[10\times {{10}^{-5}}cm\]
done
clear
D)
\[6\times {{10}^{-5}}cm\]
done
clear
View Answer play_arrow
question_answer 50) A bar magnet when placed at an angle of \[{{30}^{o}}\] to the direction of magnetic field induction of\[5\times {{10}^{-2}}T\], experiences a moment of couple\[25\times {{10}^{-6}}N-m\]. If the length of the magnet is 5 cm its pole strength is
A)
\[2\times {{10}^{-2}}A-m\]
done
clear
B)
\[5\times {{10}^{-2}}A-m\]
done
clear
C)
2 A-m
done
clear
D)
5 A-m
done
clear
View Answer play_arrow
question_answer 51) The number of moles of \[KMn{{O}_{4}}\] reduced by one mole of KI in alkaline medium is
A)
1
done
clear
B)
5
done
clear
C)
1/2
done
clear
D)
1/5
done
clear
View Answer play_arrow
question_answer 52) Value of \[x\] in potash alum,\[{{K}_{2}}S{{O}_{4}}.\,A{{l}_{x}}{{(S{{O}_{4}})}_{3}}\,.\,24{{H}_{2}}O\] is
A)
4
done
clear
B)
1
done
clear
C)
2
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 53) Ozone is used for purifying water because
A)
it dissociates and release oxygen
done
clear
B)
do not leave any foul smell like chlorine
done
clear
C)
kills bacteria, cyst, fungi and acts as a biocide
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 54) Internal energy is sum of
A)
kinetic energy and potential energy
done
clear
B)
all types of energy of the system
done
clear
C)
energy of internal system
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 55) Total volume of atoms present in a face centred cubic unit cell of a metal is (r = atomic radius)
A)
\[\frac{20}{3}\pi {{r}^{3}}\]
done
clear
B)
\[\frac{24}{3}\pi {{r}^{3}}\]
done
clear
C)
\[\frac{12}{3}\pi {{r}^{3}}\]
done
clear
D)
\[\frac{16}{3}\pi {{r}^{3}}\]
done
clear
View Answer play_arrow
question_answer 56) If two molecules of A and B having mass 100 kg and 64 kg and rate of diffusion of A is \[12\times {{10}^{-3}}\], then what will be the rate of diffusion of B?
A)
\[15\times {{10}^{-3}}\]
done
clear
B)
\[64\times {{10}^{-3}}\]
done
clear
C)
\[5\times {{10}^{-3}}\]
done
clear
D)
\[46\times {{10}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 57) Aniline is prepared in presence of Fe/HCl from
A)
benzene
done
clear
B)
nitrobenzene
done
clear
C)
dinitrobenzene
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 58) Difference between S and \[{{S}^{2-}}\] as \[{{S}^{2-}}\] has
A)
larger radii and larger size
done
clear
B)
smaller radii and larger size
done
clear
C)
larger radii and smaller size
done
clear
D)
smaller radii and smaller size
done
clear
View Answer play_arrow
question_answer 59) Which of the following is not correct?
A)
\[{{t}_{1/2}}=\frac{0.693}{k}\]
done
clear
B)
\[N={{N}_{0}}{{e}^{-kt}}\]
done
clear
C)
\[\frac{1}{N}-\frac{1}{{{N}_{0}}}=\ln \,k{{t}_{t/2}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 60) Which is not in accordance to Aufbau principle?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
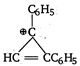
question_answer 61) \[C{{H}_{3}}C{{H}_{2}}Cl\] undergoes homolytic fission, produces
A)
\[C{{H}_{3}}\overset{\bullet }{\mathop{C}}\,{{H}_{2}}\] and \[\overset{\bullet }{\mathop{Cl}}\,\]
done
clear
B)
\[C{{H}_{3}}\overset{\oplus }{\mathop{C}}\,{{H}_{2}}\]and \[C{{l}^{O-}}\]
done
clear
C)
\[C{{H}_{3}}\overset{\oplus }{\mathop{C}}\,{{H}_{2}}\] and \[\overset{\bullet }{\mathop{Cl}}\,\]
done
clear
D)
\[C{{H}_{3}}\overset{\bullet }{\mathop{C}}\,{{H}_{2}}\]and \[C{{l}^{O-}}\]
done
clear
View Answer play_arrow
question_answer 62) C-C bond order in benzene is
A)
1
done
clear
B)
2
done
clear
C)
between 1 and 2
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 63) In colloid particles, range of diameter is
A)
1 to 100 nm
done
clear
B)
1 to 1000 cm
done
clear
C)
1 to 1000 mm
done
clear
D)
1 to 100 km
done
clear
View Answer play_arrow
question_answer 64) \[Z{{n}^{2+}}\xrightarrow{{}}Zn(s);\,\,{{E}^{o}}=-0.76\,\,V\] \[C{{u}^{2+}}\xrightarrow{{}}Cu(s);\,\,{{E}^{o}}=-0.34\,\,V\] Which of the following is spontaneous?
A)
\[Z{{n}^{2+}}+Cu\xrightarrow{{}}Zn+C{{u}^{2+}}\]
done
clear
B)
\[C{{u}^{2+}}+Zn\xrightarrow{{}}Cu+Z{{n}^{2+}}\]
done
clear
C)
\[Z{{n}^{2+}}+C{{u}^{2+}}\xrightarrow{{}}Zn+Cu\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 65) Highest electron affinity among the following is
A)
fluorine
done
clear
B)
chlorine
done
clear
C)
sulphur
done
clear
D)
xenon
done
clear
View Answer play_arrow
question_answer 66) Which of the following noble gases is most reactive?
A)
He
done
clear
B)
Ne
done
clear
C)
Ar
done
clear
D)
Xe
done
clear
View Answer play_arrow
question_answer 67) Which one of the following is a correct set with respect to molecule, hybridisation and shape?
A)
\[BeC{{l}_{2}},\,s{{p}^{2}}\], linear
done
clear
B)
\[BeC{{l}_{2}},\,s{{p}^{2}}\], triangular planar
done
clear
C)
\[BeC{{l}_{3}},\,s{{p}^{2}}\], triangular planar
done
clear
D)
\[BeC{{l}_{3}},\,s{{p}^{2}}\], tetrahedral
done
clear
View Answer play_arrow
question_answer 68) \[{{H}_{2}}S\] is not a/an
A)
reducing agent
done
clear
B)
acidic
done
clear
C)
oxidising agent
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 69) On doubling p and V with constant temperature, the equilibrium constant will
A)
remain constant
done
clear
B)
become double
done
clear
C)
become one-fourth
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 70) What is the electronic configuration of \[M{{n}^{2+}}\]?
A)
\[[Ne]\,3{{d}^{5}},4{{s}^{0}}\]
done
clear
B)
\[[Ar]\,3{{d}^{5}},4{{s}^{2}}\]
done
clear
C)
\[[Ar]\,3{{d}^{5}},4{{s}^{0}}\]
done
clear
D)
\[[Ne]\,3{{d}^{5}},4{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 71) Increase in atomic size down the group is due to
A)
increase in number of electrons
done
clear
B)
increase in number of protons and neutrons
done
clear
C)
increase in number of protons
done
clear
D)
increase in number of protons, neutrons and electrons
done
clear
View Answer play_arrow
question_answer 72) Which is tribasic acid?
A)
\[{{H}_{3}}P{{O}_{2}}\]
done
clear
B)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
C)
\[{{H}_{4}}{{P}_{2}}{{O}_{7}}\]
done
clear
D)
\[{{H}_{3}}P{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 73) Highest ionising power is exhibited by
A)
\[\alpha \]-rays
done
clear
B)
\[\beta \]-rays
done
clear
C)
\[\gamma \]-rays
done
clear
D)
X-rays
done
clear
View Answer play_arrow
question_answer 74) Blister copper is
A)
impure Cu
done
clear
B)
Cu alloy
done
clear
C)
pure Cu
done
clear
D)
Cu having 1% impurity
done
clear
View Answer play_arrow
question_answer 75) Claisen condensation is not given by
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 76) \[AgN{{O}_{3}}\] does not give precipitate with \[CHC{{l}_{3}}\] because
A)
\[CHC{{l}_{3}}\] does not ionise in water
done
clear
B)
\[AgN{{O}_{3}}\] is chemically inert
done
clear
C)
\[CHC{{l}_{3}}\] is chemically inert
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 77) \[_{90}T{{h}^{228}}\xrightarrow{{}}\,{{\,}_{83}}B{{i}^{212}}\]
A)
\[4\,\alpha ,\,\,1\beta \]
done
clear
B)
\[4\,\alpha ,\,\,2\beta \]
done
clear
C)
\[5\,\alpha ,\,\,1\beta \],
done
clear
D)
\[5\,\alpha ,\,\,2\beta \]
done
clear
View Answer play_arrow
question_answer 78) Blood cells do not shrink in blood because blood is
A)
hypotonic
done
clear
B)
isotonic
done
clear
C)
equimolar
done
clear
D)
hypertonic
done
clear
View Answer play_arrow
question_answer 79) The compound in which underlined carbon uses only its \[s{{p}^{3}}\] hybrid orbitals for bond formation is
A)
\[C{{H}_{3}}\underline{C}OOH\]
done
clear
B)
\[C{{H}_{3}}\underline{C}ON{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}\underline{C}{{H}_{2}}OH\]
done
clear
D)
\[C{{H}_{3}}\underline{C}{{H}_{2}}=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 80) t-butyl alcohol is
A)
2-methyl propan-2-ol
done
clear
B)
2-methyl propan-1-ol
done
clear
C)
3-methyl butan-1-ol
done
clear
D)
3-methyl butan-2-ol
done
clear
View Answer play_arrow
question_answer 81) Optical isomerism is shown by
A)
propanol-2
done
clear
B)
butanol-2
done
clear
C)
ethanol
done
clear
D)
methanol
done
clear
View Answer play_arrow
question_answer 82) When \[{{C}_{2}}{{H}_{2}},\,C{{H}_{4}}\] and \[{{C}_{2}}{{H}_{4}}\] passes through a test tube which have ammoniacal \[C{{u}_{2}}C{{l}_{2}}\], find out which gas comes out unaffected from test tube?
A)
\[{{C}_{2}}{{H}_{2}}\] and \[C{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{2}}\]and \[{{C}_{2}}{{H}_{4}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{4}}\] and \[C{{H}_{4}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 83) When hydrogen molecules decomposed into ifs atoms which conditions gives maximum yield of H atoms?
A)
High temperature and low pressure
done
clear
B)
Low temperature and high pressure
done
clear
C)
High temperature and high pressure
done
clear
D)
Low temperature and low pressure
done
clear
View Answer play_arrow
question_answer 84) Natural rubber is a polymer of
A)
styrene
done
clear
B)
chloroprene
done
clear
C)
\[C{{H}_{2}}=\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,-CH=C{{H}_{2}}\] or isoprene
done
clear
D)
1, 3-butadiene
done
clear
View Answer play_arrow
question_answer 85) Which of the following compounds is aromatic?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 86) The value of \[\Lambda _{eq}^{\infty }\] for \[N{{H}_{4}}Cl,\,NaOH\] and NaCI are respectively, 149.74, 248.1 and\[126.4\,\,oh{{m}^{-1}}c{{m}^{2}}e{{q}^{-1}}\]. The value of \[\Lambda _{eq}^{\infty }\] of\[N{{H}_{4}}OH\]is
A)
371.44
done
clear
B)
271.44
done
clear
C)
71.44
done
clear
D)
Cannot be predicted from given data
done
clear
View Answer play_arrow
question_answer 87) Number of atoms of He in 100 u of He (atomic wt of He is 4) are
A)
25
done
clear
B)
100
done
clear
C)
50
done
clear
D)
\[100\times 6\times {{10}^{-23}}\]
done
clear
View Answer play_arrow
question_answer 88) Which has least gold number?
A)
Gelatin
done
clear
B)
Starch
done
clear
C)
Albumin
done
clear
D)
Blood
done
clear
View Answer play_arrow
question_answer 89) In a compound C, H and N are .present in 9 : 1 : 3.5 by weight. If molecular weight of the compound is 108, then the molecular formula. of the compound is
A)
\[{{C}_{2}}{{H}_{6}}{{N}_{2}}\]
done
clear
B)
\[{{C}_{3}}{{H}_{4}}N\]
done
clear
C)
\[{{C}_{6}}{{H}_{8}}{{N}_{2}}\]
done
clear
D)
\[{{C}_{9}}{{H}_{12}}{{N}_{3}}\]
done
clear
View Answer play_arrow
question_answer 90) In the following reaction \[{{C}_{2}}{{H}_{2}}\xrightarrow[HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}]{{{H}_{2}}O}X\rightleftarrows C{{H}_{3}}CHO\], What is X?
A)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
B)
\[C{{H}_{3}}-O-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
D)
\[C{{H}_{2}}=CHOH\]
done
clear
View Answer play_arrow
question_answer 91) Which one of the following will most readily be dehydrated in acidic conditions?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 92) Which of the following solutions will have \[pH=9\] at 298K?
A)
\[1\times {{10}^{-9}}M\,HCl\] solution
done
clear
B)
\[1\times {{10}^{-5}}M\,NaOH\] solution
done
clear
C)
\[1\times {{10}^{-9}}M\,KOH\] solution
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 93)
The following data are for the decomposition of ammonium nitrite in aqueous solution. Vol. of \[{{N}_{2}}\] in cc Time (min) 6.25 10 9.00 15 11.40 20 13.65 25 35.65 Infinity
The order of reaction is
A)
zero
done
clear
B)
one
done
clear
C)
two
done
clear
D)
three
done
clear
View Answer play_arrow
question_answer 94) An alkyl halide by formation of its Grignard reagent and heating with water yields propane. What is the original alkyl halide?
A)
Methyl iodide
done
clear
B)
Ethyl iodide
done
clear
C)
Ethyl bromide
done
clear
D)
Propyl bromide
done
clear
View Answer play_arrow
question_answer 95)
The following reaction is known as
A)
Perkin reaction
done
clear
B)
Gattermann reaction
done
clear
C)
Kolbe reaction
done
clear
D)
Gattermann-aldehyde reaction
done
clear
View Answer play_arrow
question_answer 96) Aldehyde with \[N{{H}_{2}}\,.\,N{{H}_{2}}\] forms
A)
hydrazones
done
clear
B)
aniline
done
clear
C)
nitrobenzene
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 97) vant Hoff factor of \[Ca{{(N{{O}_{3}})}_{2}}\] is
A)
one
done
clear
B)
two
done
clear
C)
three
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 98) 1 mole of \[{{H}_{2}}\] and 2 moles of \[{{I}_{2}}\] are taken initially in a two litre vessel. The number of moles of \[{{H}_{2}}\] at equilibrium is 0.2. Then, the number of moles of \[{{I}_{2}}\] and HI at equilibrium are
A)
1.2, 1.6
done
clear
B)
1.8, 1.0
done
clear
C)
0.4, 2.4
done
clear
D)
0.8, 2.0
done
clear
View Answer play_arrow
question_answer 99) The volume of water to be added to \[100\,\,c{{m}^{3}}\] of 0.5 N \[{{H}_{2}}S{{O}_{4}}\] to get decinormal concentration is
A)
\[400\,\,c{{m}^{3}}\]
done
clear
B)
\[450\,\,c{{m}^{3}}\]
done
clear
C)
\[500\,\,c{{m}^{3}}\]
done
clear
D)
\[100\,\,c{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 100) 1.520 g of hydroxide of a metal on ignition gave 0.995 g of oxide. The equivalent weight of metal is
A)
1.52
done
clear
B)
0.995
done
clear
C)
190
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 101) Which is gonadotropin hormone?
A)
GH
done
clear
B)
MSH
done
clear
C)
ADH
done
clear
D)
FSH and LH
done
clear
View Answer play_arrow
question_answer 102) Bowmans capsule is found in
A)
glomerulus
done
clear
B)
uriniferous tubule
done
clear
C)
nephron
done
clear
D)
Malpighian capsule
done
clear
View Answer play_arrow
question_answer 103) During starvation what will be sequence of ending of food stuffs?
A)
Carbohydrate-fat-protein
done
clear
B)
Carbohydrate - protein -fat
done
clear
C)
Fat-protein-carbohydrate
done
clear
D)
Fat -carbohydrate - protein
done
clear
View Answer play_arrow
question_answer 104) Husband has blood group A and wife has blood group B. What is the blood group of children?
A)
A
done
clear
B)
B
done
clear
C)
AB
done
clear
D)
A, B, AB and O
done
clear
View Answer play_arrow
question_answer 105) The fruit of Solanaceae is
A)
berry or capsule
done
clear
B)
pome
done
clear
C)
legume or pod
done
clear
D)
drupe
done
clear
View Answer play_arrow
question_answer 106) \[{{O}_{3}}\] depletion is due to
A)
PAN
done
clear
B)
\[N{{O}_{x}}\]
done
clear
C)
CFCs
done
clear
D)
sulphates
done
clear
View Answer play_arrow
question_answer 107) Initiation codon is
A)
AUG
done
clear
B)
UUU
done
clear
C)
UAG
done
clear
D)
AAG
done
clear
View Answer play_arrow
question_answer 108) Mark the correct sequence of layers found in root anatomy
A)
Epiblema, cortex, endodermis, pericycle
done
clear
B)
Cortex, epiblema, pericycle, endodermis
done
clear
C)
Epiblema, cortex, pericycle, endodermis
done
clear
D)
Cortex, epiblema, endodermis, epidermis
done
clear
View Answer play_arrow
question_answer 109) Crypts of Leiberkuhn is present in
A)
small intestine
done
clear
B)
liver
done
clear
C)
stomach
done
clear
D)
colon
done
clear
View Answer play_arrow
question_answer 110) A fruit in which seed coat and fruit wall is fused known as caryopsis present in
A)
wheat
done
clear
B)
sunflower
done
clear
C)
mango
done
clear
D)
tomato
done
clear
View Answer play_arrow
question_answer 111) Which is absent in a leaf?
A)
Lenticel
done
clear
B)
Stomata
done
clear
C)
Mesophyll
done
clear
D)
Chloroplast
done
clear
View Answer play_arrow
question_answer 112) Which is a sex linked disease?
A)
Sickle cell anaemia
done
clear
B)
Haemophilia
done
clear
C)
Phenylketonuria
done
clear
D)
Albinism
done
clear
View Answer play_arrow
question_answer 113) In gyandromorphs
A)
some cells of body contain XX and some cells with genotype XY
done
clear
B)
all cells have XX genotype
done
clear
C)
all cells have XY genotype
done
clear
D)
all cells with genotype XXY
done
clear
View Answer play_arrow
question_answer 114) Catalytic converter in vehicle is used for controlling
A)
air pollution
done
clear
B)
water pollution
done
clear
C)
radioactive pollution
done
clear
D)
soil pollution
done
clear
View Answer play_arrow
question_answer 115) Bile salts help in
A)
digestion of fat
done
clear
B)
digestion and absorption of fat
done
clear
C)
digestion of protein
done
clear
D)
digestion of protein and fat
done
clear
View Answer play_arrow
question_answer 116) Spore of Funaria on germination gives rise to
A)
protonema
done
clear
B)
sporophyte
done
clear
C)
prothallus
done
clear
D)
capsule
done
clear
View Answer play_arrow
question_answer 117) Maximum numbers of vascular bundles are present in
A)
monocot stem
done
clear
B)
monocot root
done
clear
C)
dicot stem
done
clear
D)
dicotroot
done
clear
View Answer play_arrow
question_answer 118) A vascular bundles without pith is
A)
protostele
done
clear
B)
siphonostele
done
clear
C)
solenostele
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 119) Ulothrix releases zoospore during
A)
evening
done
clear
B)
morning
done
clear
C)
night
done
clear
D)
noon
done
clear
View Answer play_arrow
question_answer 120) A circulatory system, which is formed by capillaries and ends with capillaries is
A)
renal
done
clear
B)
hepatic
done
clear
C)
double circulatory system
done
clear
D)
hypophysial
done
clear
View Answer play_arrow
question_answer 121) Theory of natural selection was given by
A)
Lamarck
done
clear
B)
Darwin
done
clear
C)
Wallace
done
clear
D)
Weismann
done
clear
View Answer play_arrow
question_answer 122) In photosynthesis \[NADP{{H}_{2}}\] is formed but in respiration ifs forms during
A)
HMP
done
clear
B)
ETS
done
clear
C)
Krebs cycle
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 123) Term New Systematic was given by
A)
Julian Huxley
done
clear
B)
Bateson
done
clear
C)
Linnaeus
done
clear
D)
Darwin
done
clear
View Answer play_arrow
question_answer 124) Adrenalin and nor-adrenalin are hormones and acts as
A)
energy producing agent
done
clear
B)
food storage material
done
clear
C)
neuro transmitter
done
clear
D)
energy storing substances
done
clear
View Answer play_arrow
question_answer 125) In which cell organelles a lipoprotein covering is absent in?
A)
Ribosomes
done
clear
B)
Lysosomes
done
clear
C)
Mitochondria
done
clear
D)
Peroxisomes
done
clear
View Answer play_arrow
question_answer 126) Enveloped virus enters into host cells by
A)
injecting own nucleic acid inside host cells
done
clear
B)
by contact with cell receptor and endocytosis
done
clear
C)
by phagocytosis
done
clear
D)
fusion with the plasma membrane of host
done
clear
View Answer play_arrow
question_answer 127) On selfing RrTt we produce 400 plant, find out number of plants with genotype RrTt
A)
100
done
clear
B)
225
done
clear
C)
50
done
clear
D)
300
done
clear
View Answer play_arrow
question_answer 128) Auxin causes
A)
growth of apical bud
done
clear
B)
growth of lateral bud
done
clear
C)
seed dormancy
done
clear
D)
fall of leaf
done
clear
View Answer play_arrow
question_answer 129) Which of the following set contains only homologous organs?
A)
Whales flipper, horses forelimb, human hand
done
clear
B)
Wings of butterfly, crow and insect
done
clear
C)
Horses forelimb, insect wing, human hand
done
clear
D)
Vermiform appendix, body hair, and patella
done
clear
View Answer play_arrow
question_answer 130) Quinine used for treatment of malarial fever extracted from
A)
Atropa belladona
done
clear
B)
Cinchona officinalis
done
clear
C)
Aconitum napellus
done
clear
D)
Rauwolffia serpentine
done
clear
View Answer play_arrow
question_answer 131) Salivary gland in earthworm is found in
A)
dorsal wall of buccal cavity
done
clear
B)
ventral wall of buccal cavity
done
clear
C)
pharyngeal wall
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 132) Layers of an ovum from outside to inside is
A)
corona radiata, zoria pellucida and vitelline membrane
done
clear
B)
zona pellucida, corona radiata and vitelline membrane
done
clear
C)
vitelline membrane, zona pellucida and corona radiata
done
clear
D)
zona pellucida, vitelline membrane and corona radiate
done
clear
View Answer play_arrow
question_answer 133) Longest loop of Henie is found in
A)
kangaroo rat
done
clear
B)
opposum
done
clear
C)
rhesus monkey
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 134) Which of the following is not the character of Taenia solium?
A)
Apolysis
done
clear
B)
Proglottid
done
clear
C)
Metamerism
done
clear
D)
Strobila
done
clear
View Answer play_arrow
question_answer 135) If AAbb \[\times \] aaBB, then phenotypic ratio of its progeny will be
A)
9 : 3 : 3 : 1
done
clear
B)
1 : 2 : 1
done
clear
C)
1 : 1 : 1 : 1
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 136) Nucellus forms which of the following part of fruit?
A)
Seed coat
done
clear
B)
Perisperm
done
clear
C)
Seed
done
clear
D)
Raphe
done
clear
View Answer play_arrow
question_answer 137) Which of the following are the indicators of pollution?
A)
Lichen
done
clear
B)
Fungi
done
clear
C)
Algae
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 138) A cell swells up when kept in
A)
hypotonic solution
done
clear
B)
hypertonic solution
done
clear
C)
isotonic solution
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 139) If flowers are cut and dipped in dilute NaCI solution, then
A)
transpiration is low
done
clear
B)
endo-osmosis occurs
done
clear
C)
no bacterial growth takes .place
done
clear
D)
absorption of solute inside flower cell takes place
done
clear
View Answer play_arrow
question_answer 140) Opening and dosing of stomata is due to
A)
\[C{{a}^{2+}}\]
done
clear
B)
\[N{{a}^{+}}\]
done
clear
C)
\[{{K}^{+}}\]
done
clear
D)
\[C{{l}^{-}}\]
done
clear
View Answer play_arrow
question_answer 141) Which of the following characters are present in class Crustacea?
A)
Cephalothorax, gills and appendages
done
clear
B)
Head and thorax, gills and appendages
done
clear
C)
Cephalothorax, book gills and appendages
done
clear
D)
Head and thorax, book gills and appendages
done
clear
View Answer play_arrow
question_answer 142) Which of the following is not a concept of Lamarck?
A)
Environmental pressure causes variation
done
clear
B)
Rate and survival of organism is different due to variation
done
clear
C)
Inheritance of acquired character
done
clear
D)
If an organ is used constantly it will continuously increase its size
done
clear
View Answer play_arrow
question_answer 143) In which of the following features, Cycas resembles with angiosperms?
A)
Presence of vessels
done
clear
B)
Circinate vernation
done
clear
C)
Dichotomously branched leaves
done
clear
D)
Pollen tube is the carrier of male gametes
done
clear
View Answer play_arrow
question_answer 144) Transfer of any gene into a completely different organism can be done through
A)
genetic engineering
done
clear
B)
tissue culture
done
clear
C)
transformation
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 145) Diatoms belong to which class?
A)
Phaeophyceae
done
clear
B)
Bacillariophyceae
done
clear
C)
Chlorophyceae
done
clear
D)
Xanthophyceae
done
clear
View Answer play_arrow
question_answer 146) Pneumotaxic centre is present in
A)
cerebrum
done
clear
B)
cerebellum
done
clear
C)
medulla oblongata
done
clear
D)
pons varolii
done
clear
View Answer play_arrow
question_answer 147) Which layer develops first during embryonic development?
A)
Ectoderm
done
clear
B)
Mesoderm
done
clear
C)
Ehdoderm
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 148) During inflammation which of the following is secreted by connective tissue?
A)
Heparin
done
clear
B)
Histamine
done
clear
C)
Serotonin
done
clear
D)
Glucagon
done
clear
View Answer play_arrow
question_answer 149) Stability of ecosystem depends upon
A)
primary productivity
done
clear
B)
interchange between producers and consumers
done
clear
C)
number of producers
done
clear
D)
number of consumers
done
clear
View Answer play_arrow
question_answer 150) What is C-value paradox?
A)
Haploid DNA content
done
clear
B)
Huge variations in C-values between species
done
clear
C)
Constant C-value for all species
done
clear
D)
Diploid DNA content
done
clear
View Answer play_arrow
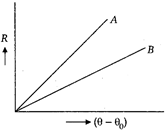 conditions. What inference do you draw from their cooling curves?
conditions. What inference do you draw from their cooling curves?