question_answer 1) A rectangular coil of 100 turns and size \[0.1\times 0.05\] m is placed perpendicular to a magnetic field of 0.1 T. The induced emf when the field drops to 0.05 T in 0.05 s is
A)
0.5V
done
clear
B)
1. 0V
done
clear
C)
1.5V
done
clear
D)
2.0V
done
clear
View Answer play_arrow
question_answer 2) A paramagnetic substance is placed in a weak magnetic field and its absolute temperature T is increased. As a result, its magnetisation
A)
increases in proportion to T
done
clear
B)
decreases in proportion to \[\frac{1}{T}\]
done
clear
C)
increases in proportion to \[{{T}^{2}}\]
done
clear
D)
decreases in proportion to \[\frac{1}{{{T}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 3) The current through a bulb is increased by 1%. Assuming that the resistance of the filament- remains unchanged the power of the bulb will
A)
increase by 1%
done
clear
B)
decrease by 1%
done
clear
C)
increase by 2%
done
clear
D)
decrease by 2%
done
clear
View Answer play_arrow
question_answer 4) You are given two resistances \[{{R}_{1}}\] and \[{{R}_{2}}\]. By using them singly, in series and in parallel, you can obtain four resistances of \[1.5\,\Omega ,\,\,2\,\,\Omega ,\,\,6\,\,\Omega \]. and \[8\,\Omega \]. The values of \[{{R}_{1}}\] and \[{{R}_{2}}\] are
A)
\[1\,\,\Omega ,\,\,7\,\,\Omega \]
done
clear
B)
\[1.5\,\,\Omega ,\,\,6.5\,\,\Omega \]
done
clear
C)
\[3\,\,\Omega ,\,\,5\,\,\Omega \]
done
clear
D)
\[2\,\,\Omega ,\,\,6\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 5) A wire of length I carrying a current I A is bent into a circle. The magnitude of the magnetic moment is
A)
\[\frac{l{{I}^{2}}}{2\pi }\]
done
clear
B)
\[\frac{l{{I}^{2}}}{4\pi }\]
done
clear
C)
\[\frac{{{l}^{2}}I}{2\pi }\]
done
clear
D)
\[\frac{{{l}^{2}}I}{4\pi }\]
done
clear
View Answer play_arrow
question_answer 6) A parallel beam of white light is reflected from a thin wedge-shaped film. The colour of the fringe at the edge of the wedge will be
A)
white
done
clear
B)
red
done
clear
C)
black
done
clear
D)
violet
done
clear
View Answer play_arrow
question_answer 7) An object is placed at the focus of a convex mirror. If its focal length is 20 cm, the distance of mirror is
A)
10 cm
done
clear
B)
20 cm
done
clear
C)
40 cm
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 8) A convex lens of glass \[(\mu =1.5)\] has a focal length of 8 cm when placed in air. What is the focal length of lens when it is immersed in water \[\left( \mu =\frac{4}{3} \right)\]?
A)
4 cm
done
clear
B)
8 cm
done
clear
C)
16cm
done
clear
D)
32cm
done
clear
View Answer play_arrow
question_answer 9) An LCR series circuit, connected to a source E, is at resonance. Then
A)
the voltage across R is zero
done
clear
B)
the voltage across R equals applied voltage
done
clear
C)
the voltage across C is zero
done
clear
D)
the voltage across L equals applied voltage
done
clear
View Answer play_arrow
question_answer 10) What will be the colour of the sky as seen from the earth if there were no atmosphere?
A)
Black
done
clear
B)
Blue
done
clear
C)
Orange
done
clear
D)
Red
done
clear
View Answer play_arrow
question_answer 11) In a single slit diffraction experiment, the width of the slit is made double its original width. Then the central maximum on the diffraction pattern will become
A)
narrower and fainter
done
clear
B)
narrower and brighter
done
clear
C)
broader and fainter
done
clear
D)
broader and brighter
done
clear
View Answer play_arrow
question_answer 12) An \[\alpha \]-particle and a deuteron projected with equal kinetic energies describe circular paths of radii \[{{r}_{1}}\] and \[{{r}_{2}}\] respectively in a uniform magnetic field. The ratio \[{{r}_{1}}/{{r}_{2}}\] is
A)
1
done
clear
B)
2
done
clear
C)
\[\frac{1}{\sqrt{2}}\]
done
clear
D)
\[\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 13) The energy in monochromatic X-rays of wavelength \[1\overset{\text{o}}{\mathop{\text{A}}}\,\] is roughly equal to
A)
\[2\times {{10}^{-15}}J\]
done
clear
B)
\[2\times {{10}^{-16}}J\]
done
clear
C)
\[2\times {{10}^{-17}}J\]
done
clear
D)
\[2\times {{10}^{-18}}J\]
done
clear
View Answer play_arrow
question_answer 14) The de-Broglie wavelength of a neutron at \[{{927}^{o}}C\] is \[\lambda \]. What will be its wavelength at\[{{27}^{o}}C\]?
A)
\[\frac{\lambda }{2}\]
done
clear
B)
\[\lambda \]
done
clear
C)
\[2\lambda \]
done
clear
D)
\[4\lambda \]
done
clear
View Answer play_arrow
question_answer 15) A telescope has an objective of focal length 100 cm and an eye-piece of focal length 5 cm. What is the magnifying power of the telescope when it is in normal adjustment?
A)
0.2
done
clear
B)
2.0
done
clear
C)
20
done
clear
D)
200
done
clear
View Answer play_arrow
question_answer 16) An isotropic source of 2 candela produces a light flux equal to
A)
\[2\pi \] lumen
done
clear
B)
\[4\pi \] lumen
done
clear
C)
\[6\pi \] lumen
done
clear
D)
\[8\pi \] lumen
done
clear
View Answer play_arrow
question_answer 17) No photoelectrons are emitted from a metal if the wavelength of the light exceeds 600 nm. The work function of the metal is approximately equal to
A)
\[3\times {{10}^{-16}}J\]
done
clear
B)
\[3\times {{10}^{-19}}J\]
done
clear
C)
\[3\times {{10}^{-20}}J\]
done
clear
D)
\[3\times {{10}^{-22}}J\]
done
clear
View Answer play_arrow
question_answer 18) In Rutherfords a-particle experiment with thin gold foil, 8100 scintillations per minute are observed at an angle of \[{{60}^{o}}\]. The number of scintillations per minute at an angle of \[{{120}^{o}}\] will be
A)
900
done
clear
B)
2025
done
clear
C)
32400
done
clear
D)
4050
done
clear
View Answer play_arrow
question_answer 19)
Which of the following gates has the truth table? A B C 0 0 1 1 0 1 0 1 1 1 1 0
A)
NAND
done
clear
B)
NOR
done
clear
C)
XOR
done
clear
D)
AND
done
clear
View Answer play_arrow
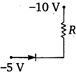
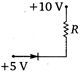
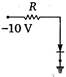
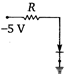
question_answer 20) Which of the junction diodes shown below are forward biased?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 21) A uniform rod of mass m and length I is held inclined at an angle of \[{{60}^{o}}\] with the vertical. What will be the potential energy of the rod in this position?
A)
Zero
done
clear
B)
\[\frac{mgl}{4}\]
done
clear
C)
\[\frac{mgl}{2}\]
done
clear
D)
\[mgl\]
done
clear
View Answer play_arrow
question_answer 22) A sphere rolls down an inclined plane without slipping. What fraction of its total energy is rotational?
A)
\[\frac{2}{7}\]
done
clear
B)
\[\frac{3}{7}\]
done
clear
C)
\[\frac{4}{7}\]
done
clear
D)
\[\frac{5}{7}\]
done
clear
View Answer play_arrow
question_answer 23) From the top of a building 40 m tall, a boy projects a stone vertically upwards with an initial velocity 10 \[m{{s}^{-1}}\] such that it eventually falls to the ground. After how long will the stone strike the ground? Take \[g=10\,\,m{{s}^{-2}}\]
A)
1 s
done
clear
B)
2 s
done
clear
C)
3 s
done
clear
D)
4 s
done
clear
View Answer play_arrow
question_answer 24) A 150 m long train is travelling from east to west at a speed of 20 \[m{{s}^{-1}}\]. A bird is flying from west to east at a speed of 5 \[m{{s}^{-1}}\]. How long will the bird take to cross the train?
A)
6s
done
clear
B)
7.5 s
done
clear
C)
10 s
done
clear
D)
30 s
done
clear
View Answer play_arrow
question_answer 25) An elastic spring has a length \[{{l}_{1}}\] when it is stretched with a force of 2 N and a length of \[{{l}_{2}}\]when it is stretched with a force of 3N. What will be the length of the spring if it is stretched with force of 5N?
A)
\[({{l}_{1}}+{{l}_{2}})\]
done
clear
B)
\[\frac{1}{2}({{l}_{1}}+{{l}_{2}})\]
done
clear
C)
\[(3{{l}_{2}}-2{{l}_{1}})\]
done
clear
D)
\[(3{{l}_{1}}-2{{l}_{2}})\]
done
clear
View Answer play_arrow
question_answer 26) The density of water at the surface of ocean is\[\rho \]. If the bulk modulus of water is B, what is the density of ocean water at a depth where the pressure is \[n{{\rho }_{0}}\], where \[{{p}_{0}}\] is the atmospheric pressure?
A)
\[\frac{\rho B}{B-(n-1){{p}_{0}}}\]
done
clear
B)
\[\frac{\rho B}{B+(n-1){{p}_{0}}}\]
done
clear
C)
\[\frac{\rho B}{B-n{{p}_{0}}}\]
done
clear
D)
\[\frac{\rho B}{B+n{{p}_{0}}}\]
done
clear
View Answer play_arrow
question_answer 27) What is the torque of force \[\vec{F}=3\,\hat{i}+\hat{j}+5\hat{k}\]acting at point whose position vector is\[\vec{r}=7\,\hat{i}+3\hat{j}+\hat{k}\]?
A)
\[14\,\hat{i}-38\hat{j}+16\,\hat{k}\]
done
clear
B)
\[4\,\hat{i}+4\hat{j}+6\,\hat{k}\]
done
clear
C)
\[14\,\hat{i}+38\hat{j}-16\,\hat{k}\]
done
clear
D)
\[-21\,\hat{i}+3\hat{j}+5\,\hat{k}\]
done
clear
View Answer play_arrow
question_answer 28) Two satellites of masses 3M and M orbit the earth in circular orbits of radii rand3r respectively. The ratio of their speeds is
A)
1 : 1
done
clear
B)
\[\sqrt{3}\,:1\]
done
clear
C)
3 : 1
done
clear
D)
9 : 1
done
clear
View Answer play_arrow
question_answer 29) The dimensions of Reynoldss constant are
A)
\[[{{M}^{0}}{{L}^{0}}{{T}^{0}}]\]
done
clear
B)
\[[M{{L}^{-1}}{{T}^{-1}}]\]
done
clear
C)
\[[M{{L}^{-1}}{{T}^{-2}}]\]
done
clear
D)
\[[M{{L}^{-2}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 30) The surface tension of soap solution is or. What is the work done in blowing soap bubble of radius r?
A)
\[\pi {{r}^{2}}\sigma \]
done
clear
B)
\[2\pi {{r}^{2}}\sigma \]
done
clear
C)
\[4\pi {{r}^{2}}\sigma \]
done
clear
D)
\[8\pi {{r}^{2}}\sigma \]
done
clear
View Answer play_arrow
question_answer 31) The ratio of rms speed of \[{{O}_{2}}\] to \[{{H}_{2}}\] is
A)
\[\frac{1}{4}\]
done
clear
B)
4
done
clear
C)
2
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
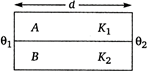
question_answer 32)
Two rods A and B of different materials are welded together as shown in figure. If their thermal conductivities are \[{{K}_{1}}\] and \[{{K}_{2}}\] the thermal conductivity of the composite rod will be
A)
\[\frac{1}{2}\,pV\]
done
clear
B)
\[pV\]
done
clear
C)
\[2pV\]
done
clear
D)
\[4pV\]
done
clear
View Answer play_arrow
question_answer 33) The wavelength of the radiation emitted by a body depends upon
A)
the nature of its surface
done
clear
B)
the area of its surface
done
clear
C)
the temperature of its surface
done
clear
D)
All the above factors
done
clear
View Answer play_arrow
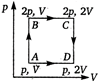
question_answer 34)
An ideal monoatomic gas is taken around the cycle ABCDA as shown in the p - V diagram. The work done during the cycle is given by
A)
\[\frac{1}{2}pV\]
done
clear
B)
\[pV\]
done
clear
C)
\[2pV\]
done
clear
D)
\[4pV\]
done
clear
View Answer play_arrow
question_answer 35) An ideal gas at pressure p is adiabatically compressed so that its density becomes n times the initial value The final pressure of the gas will be \[\left( \gamma =\frac{{{C}_{p}}}{{{C}_{V}}} \right)\]
A)
\[{{n}^{\gamma }}p\]
done
clear
B)
\[{{n}^{-\gamma }}p\]
done
clear
C)
\[{{n}^{(\gamma -1)}}p\]
done
clear
D)
\[{{n}^{(\gamma -1)}}p\]
done
clear
View Answer play_arrow
question_answer 36) The displacement of a travelling wave is given by \[y=2\,\cos \,2\pi (10t-0.008x+0.35)\] Where, \[x\]and y are in centimetre and t in second. What is the phase difference between oscillatory motion at two points separated by a distance of 4 m?
A)
\[2\,\,\pi \]
done
clear
B)
\[4\,\,\pi \]
done
clear
C)
\[6\,\,\pi \]
done
clear
D)
\[8\,\,\pi \]
done
clear
View Answer play_arrow
question_answer 37) A bimetallic strip consists of brass and iron when it is heated it bends into an arc with brass on the convex and iron on the concave side of the arc. This happens because
A)
brass has a higher specific heat capacity than iron
done
clear
B)
density of brass is more than that of iron
done
clear
C)
it is easier to bend an iron strip than a brass strip of the same size
done
clear
D)
brass has a higher coefficient of linear expansion than iron
done
clear
View Answer play_arrow
question_answer 38) Three sound waves of equal amplitudes have frequencies (v - 1), v, (v + 1). They superpose to give beats. The number of beats produced per second will be -
A)
\[v\]
done
clear
B)
\[v/2\]
done
clear
C)
2
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 39) Which of the following functions does not represent a stationary wave? Here a, b and c are constants.
A)
\[y=a\cos bx\sin ct\]
done
clear
B)
\[y=a\sin bx\cos ct\]
done
clear
C)
\[y=a\sin \,(bx+ct)\]
done
clear
D)
\[y=a\sin \,(bx+ct)+a\sin \,(bx-ct)\]
done
clear
View Answer play_arrow
question_answer 40) The velocity of sound is greatest in
A)
water
done
clear
B)
air
done
clear
C)
vacuum
done
clear
D)
steel
done
clear
View Answer play_arrow
question_answer 41) Due to Doppler effect, the shift in wavelength observed is 0.1, \[\overset{o}{\mathop{A}}\,\], for a star producing wavelength 6000 \[\overset{o}{\mathop{A}}\,\]. The velocity of recession of
A)
2.5 \[km{{s}^{-1}}\]
done
clear
B)
10 \[km{{s}^{-1}}\]
done
clear
C)
5 kms
done
clear
D)
20 \[km{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 42) A body executing linear simple harmonic motion has a velocity of 3 \[m{{s}^{-1}}\] when its displacement is 4 cm and a velocity of 4 \[m{{s}^{-1}}\]when its displacement is 3 cm. What is the amplitude of oscillation?
A)
5 cm
done
clear
B)
7.5 cm
done
clear
C)
10 cm
done
clear
D)
12.5 cm
done
clear
View Answer play_arrow
question_answer 43)
Two masses \[{{m}_{1}}\] and \[{{m}_{2}}\] are suspended together by a massless spring of force constant k, as shown in figure. When the masses are in equilibrium, mass \[{{m}_{1}}\], is removed without disturbing the system. The angular frequency of oscillation of mass \[{{m}_{2}}\] is
A)
\[\sqrt{\frac{k}{{{m}_{2}}}}\]
done
clear
B)
\[\sqrt{\frac{k}{m1}}\]
done
clear
C)
\[\sqrt{\frac{k{{m}_{1}}}{m_{2}^{2}}}\]
done
clear
D)
\[\sqrt{\frac{k{{m}_{2}}}{m_{1}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 44) The intensity level of a sound wave is defined by an arbitrary scale. The zero of the scale is taken at the sound wave intensity
A)
\[1\times {{10}^{-10}}W{{m}^{-2}}\]
done
clear
B)
\[1\times {{10}^{-12}}W{{m}^{-2}}\]
done
clear
C)
\[1\times {{10}^{-14}}W{{m}^{-2}}\]
done
clear
D)
\[1\times {{10}^{-16}}W{{m}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 45) A pendulum bob of mass m carrying a charge q is at rest with its string making an angle 9 with the vertical in a uniform horizontal electric field E, The tension in the string is
A)
\[\frac{mg}{\sin \theta }\] and \[\frac{qE}{\cos \theta }\]
done
clear
B)
\[\frac{mg}{\cos \theta }\] and \[\frac{qE}{\sin \theta }\]
done
clear
C)
\[\frac{qE}{mg}\]
done
clear
D)
\[\frac{mg}{qE}\]
done
clear
View Answer play_arrow
question_answer 46) A galvanometer of resistance \[10\,\,\Omega \]. gives full-scale deflection when 1 mA current passes through it. The resistance required to convert it into a voltmeter reading upto 2.5 V is
A)
\[24.9\,\,\Omega \]
done
clear
B)
\[249\,\,\Omega \]
done
clear
C)
\[2490\,\,\Omega \]
done
clear
D)
\[24900\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 47) The current inside an electrolytic cell is carried by
A)
positive ions
done
clear
B)
negative ions
done
clear
C)
both positive and negative ions
done
clear
D)
electrons
done
clear
View Answer play_arrow
question_answer 48) The electric potential V (in volt) varies with x (in metre) according to the relation\[V=(5+4{{x}^{2}})\]. The force experienced by a negative charge of \[2\times {{10}^{-6}}C\] located at x = 0.5 m is
A)
\[2\times {{10}^{-6}}N\]
done
clear
B)
\[4\times {{10}^{-6}}N\]
done
clear
C)
\[6\times {{10}^{-6}}N\]
done
clear
D)
\[8\times {{10}^{-6}}N\]
done
clear
View Answer play_arrow
question_answer 49) A parallel plate capacitor of plate area A has a charge Q. The force on each pi ate of the capacitor is
A)
\[\frac{2{{Q}^{2}}}{{{\varepsilon }_{0}}A}\]
done
clear
B)
\[\frac{{{Q}^{2}}}{{{\varepsilon }_{0}}A}\]
done
clear
C)
\[\frac{{{Q}^{2}}}{2{{\varepsilon }_{0}}A}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 50) In Millikans oil drop experiment, an oil drop is observed to move vertically upwards. The upward motion of the drop is due to
A)
gravity
done
clear
B)
viscosity
done
clear
C)
buoyancy
done
clear
D)
electric field
done
clear
View Answer play_arrow
question_answer 51) Which one of these is an important polysaccharide present in plants?
A)
Glucose
done
clear
B)
Glycogen
done
clear
C)
Sucrose
done
clear
D)
Starch
done
clear
View Answer play_arrow
question_answer 52) Cannizaro reaction would be given by
A)
\[{{C}_{6}}{{H}_{5}}CHO\]
done
clear
B)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}CO{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 53) \[C{{H}_{3}}C\equiv CH\xrightarrow[+H{{g}^{2+}},{{60}^{o}}C]{{{H}_{2}}S{{O}_{4}}}?\]
A)
\[C{{H}_{3}}CHO\]
done
clear
B)
\[C{{H}_{3}}COOH\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 54) In which of the following can peroxide effect operate?
A)
\[C{{H}_{3}}C{{H}_{2}}CH=C{{H}_{2}}+HCl\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}CH=C{{H}_{2}}+HBr\]
done
clear
C)
\[C{{H}_{3}}CH=CH\,.\,C{{H}_{3}}+HBr\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}CH=C{{H}_{2}}+HI\]
done
clear
View Answer play_arrow
question_answer 55) The term quaternary structure is used in the context of
A)
carbohydrates
done
clear
B)
vitamins
done
clear
C)
proteins
done
clear
D)
nucleic acids
done
clear
View Answer play_arrow
question_answer 56) Aspirin is
A)
antibiotic
done
clear
B)
sedative
done
clear
C)
tranquilizer
done
clear
D)
antipyretic
done
clear
View Answer play_arrow
question_answer 57) The following is a coordination compound of iron
A)
chlorophyll
done
clear
B)
Mohrs salt
done
clear
C)
haemoglobin
done
clear
D)
vitamin \[{{B}_{12}}\]
done
clear
View Answer play_arrow
question_answer 58) This metal is extracted from its ore by formation of a cyanide complex
A)
Ag
done
clear
B)
Cu
done
clear
C)
Al
done
clear
D)
Zn
done
clear
View Answer play_arrow
question_answer 59) Smoke is an example of
A)
emulsion
done
clear
B)
aerosol
done
clear
C)
gel
done
clear
D)
foam
done
clear
View Answer play_arrow
question_answer 60) Propan-2-ol + ethanoic acid \[\xrightarrow{{}}\]?
A)
\[{{(C{{H}_{3}})}_{2}}CHCOOC{{H}_{3}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}COOCH{{(C{{H}_{3}})}_{2}}\]
done
clear
C)
\[C{{H}_{3}}COOC{{H}_{2}}C{{H}_{3}}\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}CHCOOC{{H}_{2}}C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 61) Which of the following gives RNC when treated with \[CHC{{l}_{3}}\] and KOH?
A)
\[RN{{H}_{2}}\]
done
clear
B)
\[{{R}_{2}}NH\]
done
clear
C)
\[{{R}_{4}}{{N}^{+}}C{{l}^{-}}\]
done
clear
D)
\[{{R}_{3}}N\]
done
clear
View Answer play_arrow
question_answer 62) Which of the following is a soap?
A)
Sodium acetate
done
clear
B)
Magnesium butyrate
done
clear
C)
Sodium palmitate
done
clear
D)
Palmitic acid
done
clear
View Answer play_arrow
question_answer 63) The C-C bond dissociation energy in kcal/mol is
A)
8.1
done
clear
B)
0.81
done
clear
C)
81
done
clear
D)
810
done
clear
View Answer play_arrow
question_answer 64) A compound has four \[s{{p}^{3}}\] hybridised covalent bonds. Its shape is
A)
linear
done
clear
B)
square planar
done
clear
C)
octahedral
done
clear
D)
tetrahedral
done
clear
View Answer play_arrow
question_answer 65) The resonance energy of benzene in kcal/mol is about
A)
\[\sim 20\]
done
clear
B)
\[\sim 40\]
done
clear
C)
\[\sim 60\]
done
clear
D)
\[\sim 80\]
done
clear
View Answer play_arrow
question_answer 66) 1 mole of \[C{{O}_{2}}\] contains
A)
\[6\times {{10}^{23}}\] atoms of C
done
clear
B)
\[6\times {{10}^{23}}\] atoms of O
done
clear
C)
\[18\times {{10}^{23}}\] molecules of \[C{{O}_{2}}\]
done
clear
D)
3 g-atoms of \[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 67) Rutherfords famous experiment with \[\alpha \]-particles used this metal
A)
Ni
done
clear
B)
Au
done
clear
C)
Fe
done
clear
D)
Zn
done
clear
View Answer play_arrow
question_answer 68) The charge on an electron in Coulombs is
A)
\[1.602\times {{10}^{-19}}\]
done
clear
B)
\[1.062\times {{10}^{-19}}\]
done
clear
C)
\[1.620\times {{10}^{-19}}\]
done
clear
D)
\[1.006\times {{10}^{-19}}\]
done
clear
View Answer play_arrow
question_answer 69) The number of subshells for a shell with principal quantum number n is
A)
n
done
clear
B)
n + 1
done
clear
C)
n -1
done
clear
D)
\[2{{n}^{2}}\]
done
clear
View Answer play_arrow
question_answer 70) Which of the following pairs of atomic numbers represents s-block elements?
A)
7, 15
done
clear
B)
5, 12
done
clear
C)
9, 17
done
clear
D)
3, 11
done
clear
View Answer play_arrow
question_answer 71) For a system in equilibrium
A)
\[\Delta G=0\]
done
clear
B)
\[\Delta G>0\]
done
clear
C)
\[\Delta G<0\]
done
clear
D)
\[\Delta H=0\]
done
clear
View Answer play_arrow
question_answer 72) Which of the following will produce the highest rise in temperature?
A)
67 mL of 1 M NaOH + 33 mL of 0.5 M \[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
33 mL of 1 M NaOH + 67 mL of 0.5 M \[{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
40 mL of 1 M NaOH + 60 mL of 0.5 M \[{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
50 mL of 1 M NaOH + 50 mL of 0.5 M \[{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 73) The standard for atomic mass is
A)
\[_{1}^{1}H\]
done
clear
B)
\[_{6}^{12}C\]
done
clear
C)
\[_{6}^{14}C\]
done
clear
D)
\[_{8}^{14}O\]
done
clear
View Answer play_arrow
question_answer 74) Which of the following involves absorption of energy?
A)
\[Cl+{{e}^{-}}\xrightarrow{{}}C{{l}^{-}}\]
done
clear
B)
\[{{O}^{-}}+{{e}^{-}}\xrightarrow{{}}{{O}^{2-}}\]
done
clear
C)
\[O+{{e}^{-}}\xrightarrow{{}}{{O}^{-}}\]
done
clear
D)
\[S+{{e}^{-}}\xrightarrow{{}}{{S}^{-}}\]
done
clear
View Answer play_arrow
question_answer 75) The successive ionisation energies of an element are 800, 2,000, 3,600, 25,000 and 32,000 kJ/mol. The number of valence electrons are
A)
5
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 76) The first order rate constant for the decomposition of \[{{N}_{2}}{{O}_{5}}\] is \[6.2\times {{10}^{-4}}{{s}^{-1}}\]. The half-life in seconds is nearly
A)
1117.7
done
clear
B)
111.7
done
clear
C)
223
done
clear
D)
160
done
clear
View Answer play_arrow
question_answer 77) The rate constant of a reaction is\[1.2\times {{10}^{-2}}mo{{l}^{-2}}{{L}^{2}}{{s}^{-1}}\]. The order of the reaction is
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 78) The oxidation number of 0 in \[O{{F}_{2}}\] is
A)
-2
done
clear
B)
+1
done
clear
C)
+2
done
clear
D)
-1
done
clear
View Answer play_arrow
question_answer 79) The correct relationship is
A)
\[{{K}_{C}}=n\times {{K}_{p}}\]
done
clear
B)
\[{{K}_{p}}={{K}_{C}}{{(RT)}^{\Delta {{n}_{g}}}}\]
done
clear
C)
\[{{K}_{p}}=n\times {{K}_{C}}\]
done
clear
D)
\[{{K}_{C}}-{{K}_{p}}={{(RT)}^{\Delta N}}\]
done
clear
View Answer play_arrow
question_answer 80) For the reaction, \[2B+AC\], the equilibrium constant is
A)
\[\frac{[A]\,{{[B]}^{3}}}{[C]}\]
done
clear
B)
\[\frac{[C]}{[A]\,[2B]}\]
done
clear
C)
\[\frac{[C]}{[A]\,{{[B]}^{2}}}\]
done
clear
D)
\[\frac{[A]\,[B]}{[C]}\]
done
clear
View Answer play_arrow
question_answer 81) A buffer contains equal concentrations of \[{{X}^{-}}\]and HX. The \[{{K}_{a}}\] for HX is \[{{10}^{-8}}\]. The pH of the buffer is
A)
3
done
clear
B)
8
done
clear
C)
7
done
clear
D)
11
done
clear
View Answer play_arrow
question_answer 82) The metal which gives \[{{H}_{2}}\] on treatment with acid as well as NaOH is
A)
Fe
done
clear
B)
Cu
done
clear
C)
Zn
done
clear
D)
Hg
done
clear
View Answer play_arrow
question_answer 83) Which is isoelectronic with hydride ion?
A)
He
done
clear
B)
\[H{{e}^{+}}\]
done
clear
C)
Li
done
clear
D)
\[{{H}^{+}}\]
done
clear
View Answer play_arrow
question_answer 84) A solution of Na in liquid \[N{{H}_{3}}\] is
A)
green
done
clear
B)
blue
done
clear
C)
pink
done
clear
D)
yellow
done
clear
View Answer play_arrow
question_answer 85) The oxide which gives \[{{H}_{2}}{{O}_{2}}\] on treatment with dilute acid is
A)
\[Pb{{O}_{2}}\]
done
clear
B)
\[Ti{{O}_{2}}\]
done
clear
C)
\[Mn{{O}_{2}}\]
done
clear
D)
\[N{{a}_{2}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 86) During the electrolysis of fused NaCI, this happens at cathode
A)
oxidation of \[N{{a}^{+}}\]
done
clear
B)
reduction of \[N{{a}^{+}}\]
done
clear
C)
reduction of \[C{{l}^{-}}\]
done
clear
D)
oxidation of \[C{{l}^{-}}\]
done
clear
View Answer play_arrow
question_answer 87) Which of the following is a coloured gas?
A)
\[{{N}_{2}}O\]
done
clear
B)
NO
done
clear
C)
\[{{H}_{2}}\]
done
clear
D)
\[N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 88) This compound gives off \[{{O}_{2}}\] on moderate heating
A)
HgO
done
clear
B)
ZnO
done
clear
C)
CaO
done
clear
D)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 89) Plaster of Paris is hardened by
A)
liberating \[C{{O}_{2}}\]
done
clear
B)
hydration
done
clear
C)
dehydration
done
clear
D)
changing into \[CaC{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 90) The least ionic chloride is formed by
A)
Mg
done
clear
B)
Ca
done
clear
C)
Be
done
clear
D)
Sr
done
clear
View Answer play_arrow
question_answer 91) Aluminium bronze has
A)
90% Cu
done
clear
B)
50% Ni
done
clear
C)
90% Sn
done
clear
D)
50% Cu
done
clear
View Answer play_arrow
question_answer 92) In the thermite welding, this is used
A)
Ca
done
clear
B)
Al + Fe
done
clear
C)
Fe
done
clear
D)
\[Al+F{{e}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 93) \[{{K}_{2}}C{{O}_{3}}\] cannot be prepared by Solvay-ammonia process because
A)
\[{{K}_{2}}C{{O}_{3}}\] is fairly soluble in water
done
clear
B)
it has no water of crystallisation
done
clear
C)
\[KHC{{O}_{3}}\] is highly soluble in water
done
clear
D)
\[{{K}_{2}}C{{O}_{3}}\] decomposes in \[{{H}_{2}}O\])
done
clear
View Answer play_arrow
question_answer 94) Glaubers salt is
A)
\[N{{a}_{2}}S{{O}_{4}}\,.\,10{{H}_{2}}O\]
done
clear
B)
\[FeS{{O}_{4}}\,.\,7{{H}_{2}}O\]
done
clear
C)
\[ZnS{{O}_{4}}\,.\,7{{H}_{2}}O\]
done
clear
D)
\[MgS{{O}_{4}}\,.\,7{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 95) The salt responsible for permanent hardness of \[{{H}_{2}}O\] is
A)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
B)
\[MgHC{{O}_{3}}\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[MgC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 96) Which of the following carbonium ion is most stable?
A)
\[{{C}_{6}}{{H}_{5}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}\overset{+}{\mathop{C}}\,\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}\overset{+}{\mathop{C}}\,H\]
done
clear
D)
\[C{{H}_{3}}\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 97) Which of the following reacts in presence of anhydrous \[AlC{{l}_{3}}\] to give acetophenone?
A)
\[{{C}_{6}}{{H}_{6}}+C{{H}_{3}}COC{{H}_{3}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}OH+C{{H}_{3}}COC{{H}_{3}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}OH+C{{H}_{3}}COOH\]
done
clear
D)
\[{{C}_{6}}{{H}_{6}}+C{{H}_{3}}COCl\]
done
clear
View Answer play_arrow
question_answer 98) The isomers which can be interconverted through rotation around a single bond are
A)
conformers
done
clear
B)
diastereoisomers
done
clear
C)
enantiomers
done
clear
D)
position isomers
done
clear
View Answer play_arrow
question_answer 99) Phenol is the component of this fraction upon fractional distillation of petroleum
A)
light oil
done
clear
B)
middle Oil
done
clear
C)
heavy oil
done
clear
D)
pitch
done
clear
View Answer play_arrow
question_answer 100) Ethyl chloride on treatment with alcoholic alkali gives
A)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
D)
\[C{{H}_{2}}=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 101) Total number of pairs of cranial nerves in human is
A)
12
done
clear
B)
10
done
clear
C)
6
done
clear
D)
20
done
clear
View Answer play_arrow
question_answer 102) The target organ for vasopressin is
A)
thyroid
done
clear
B)
kidneys
done
clear
C)
gonad
done
clear
D)
mammary glands
done
clear
View Answer play_arrow
question_answer 103) Dahlia is propagated through
A)
roots
done
clear
B)
stem
done
clear
C)
bud
done
clear
D)
leaf
done
clear
View Answer play_arrow
question_answer 104) Suspension of plant growth due to exogenous control is called
A)
vivipary
done
clear
B)
germination
done
clear
C)
quiescence
done
clear
D)
dormancy
done
clear
View Answer play_arrow
question_answer 105) These induce production of enzymes during seed germination
A)
auxins
done
clear
B)
cytokinins
done
clear
C)
ethylene
done
clear
D)
gibberellins
done
clear
View Answer play_arrow
question_answer 106) Many xerophytes accumulate this in response to stress
A)
glucose
done
clear
B)
proline
done
clear
C)
lysine
done
clear
D)
fatty acids
done
clear
View Answer play_arrow
question_answer 107) Whales show this adaptation strategy
A)
mimicry
done
clear
B)
hibernation
done
clear
C)
migration
done
clear
D)
camouflage
done
clear
View Answer play_arrow
question_answer 108) The bone of the knee cap is called
A)
patella
done
clear
B)
femur
done
clear
C)
tibia
done
clear
D)
fibula
done
clear
View Answer play_arrow
question_answer 109) Osteoporesis is a disorder of
A)
kidney
done
clear
B)
muscle
done
clear
C)
bones
done
clear
D)
heart
done
clear
View Answer play_arrow
question_answer 110) Smell is a function of this lobe
A)
frontal
done
clear
B)
parietal
done
clear
C)
occipital
done
clear
D)
temporal
done
clear
View Answer play_arrow
question_answer 111) The site of glycolysis is
A)
ribosomes
done
clear
B)
mitochondria
done
clear
C)
nucleus
done
clear
D)
cytoplasm
done
clear
View Answer play_arrow
question_answer 112) In respiration, the released energy forms
A)
AMP
done
clear
B)
ADP
done
clear
C)
ATP
done
clear
D)
Glucose
done
clear
View Answer play_arrow
question_answer 113) The number of bases found in RNA are
A)
4
done
clear
B)
3
done
clear
C)
5
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 114) The pseudouridine loop is present in
A)
DNA
done
clear
B)
tRNA
done
clear
C)
mRNA
done
clear
D)
rRNA
done
clear
View Answer play_arrow
question_answer 115) This is a structural polysaccharide
A)
starch
done
clear
B)
chitin
done
clear
C)
amylase
done
clear
D)
glycogen
done
clear
View Answer play_arrow
question_answer 116) 70S ribosomes have two units. These are
A)
50S + 30S
done
clear
B)
40S + 30S
done
clear
C)
50S + 20S
done
clear
D)
40S + 60S
done
clear
View Answer play_arrow
question_answer 117) The outer face of the outer membrane of Gram negative bacteria contains
A)
peptidoglycans
done
clear
B)
teichqic acids
done
clear
C)
lipopolysaccharides
done
clear
D)
mycoic acid
done
clear
View Answer play_arrow
question_answer 118) These impart rigidity to plasma membrane
A)
phospholipids
done
clear
B)
sterols
done
clear
C)
proteins
done
clear
D)
carbohydrates
done
clear
View Answer play_arrow
question_answer 119) Which one of the following has least similar characters?
A)
Family
done
clear
B)
Class
done
clear
C)
Genus
done
clear
D)
Species
done
clear
View Answer play_arrow
question_answer 120) Blue green algae belong to this kingdom
A)
Plantae
done
clear
B)
Protista
done
clear
C)
Fungi
done
clear
D)
Monera
done
clear
View Answer play_arrow
question_answer 121) Which one of the following has characters of both plant and animal?
A)
Bacteria
done
clear
B)
Mycoplasma
done
clear
C)
Euglena
done
clear
D)
Paramecium
done
clear
View Answer play_arrow
question_answer 122) The shape of the Micrococcus bacteria is
A)
rod
done
clear
B)
spiral
done
clear
C)
oval
done
clear
D)
coil
done
clear
View Answer play_arrow
question_answer 123) What is called Dahlia starch?
A)
Cellulose
done
clear
B)
Chitin
done
clear
C)
Inulin
done
clear
D)
Glycogen
done
clear
View Answer play_arrow
question_answer 124) The fastestenzyme among the following is
A)
urease
done
clear
B)
trypsin
done
clear
C)
amylase
done
clear
D)
carbonic anhydrase
done
clear
View Answer play_arrow
question_answer 125) Km value of an enzyme is substrate concentration at
A)
\[\frac{1}{4}\,{{V}_{\max }}\]
done
clear
B)
\[2\,{{V}_{\max }}\]
done
clear
C)
\[\frac{1}{2}\,{{V}_{\max }}\]
done
clear
D)
\[{{V}_{\max }}\]
done
clear
View Answer play_arrow
question_answer 126) This fungus is not edible
A)
Agaricus
done
clear
B)
Toadstool
done
clear
C)
Puffballs
done
clear
D)
Morchella
done
clear
View Answer play_arrow
question_answer 127) Aflatoxin is produced by a
A)
virus
done
clear
B)
bacteria
done
clear
C)
Ascomycota fungi
done
clear
D)
Basidiomycota fungi
done
clear
View Answer play_arrow
question_answer 128) Number of cotyledons in Pinus seed are
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
many
done
clear
View Answer play_arrow
question_answer 129) These are called amphibians of plant kingdom
A)
Bryophytes
done
clear
B)
Brown algae
done
clear
C)
Green algae
done
clear
D)
Riccia
done
clear
View Answer play_arrow
question_answer 130) Pigment common to all algae is
A)
alginate
done
clear
B)
carotenoids
done
clear
C)
phycocyanin
done
clear
D)
chlorophyll
done
clear
View Answer play_arrow
question_answer 131) Mycoplasmas are sensitive to
A)
tetracyclines
done
clear
B)
penicillin
done
clear
C)
sugars
done
clear
D)
amino acids
done
clear
View Answer play_arrow
question_answer 132) This bacteria produces a well known insecticide
A)
B. thuringiensis
done
clear
B)
B. polymyxa
done
clear
C)
B. brevis
done
clear
D)
B. licheniformis
done
clear
View Answer play_arrow
question_answer 133) Nutrition in Amoeba is
A)
holophytic
done
clear
B)
parasitic
done
clear
C)
holozoic
done
clear
D)
saprobic
done
clear
View Answer play_arrow
question_answer 134) Which one is an antinutritional factor?
A)
Lysine
done
clear
B)
Tryptophan
done
clear
C)
Albumin
done
clear
D)
Glucosinolates
done
clear
View Answer play_arrow
question_answer 135) Xanthomonas citri is a pathogen responsible for a disease in this crop.
A)
potato
done
clear
B)
Citrus
done
clear
C)
tomato
done
clear
D)
wheat
done
clear
View Answer play_arrow
question_answer 136) Flooding of the field is an effective control measure for this type of pathogen
A)
Fungi
done
clear
B)
Vacteria
done
clear
C)
Nematode
done
clear
D)
Viruses
done
clear
View Answer play_arrow
question_answer 137) Protoplasts of a plant cell are produced by treatment with
A)
Cellulase
done
clear
B)
Pectinase
done
clear
C)
PEG
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 138) Processing of single cell protein generally involves removal of
A)
nucleic acids
done
clear
B)
fats
done
clear
C)
carbohydrates
done
clear
D)
oils
done
clear
View Answer play_arrow
question_answer 139) An animal, which comes out at night and hides during the day is
A)
diurnal
done
clear
B)
nocturnal
done
clear
C)
cursorial
done
clear
D)
arboreal
done
clear
View Answer play_arrow
question_answer 140) Sponges belong to this phylum
A)
Mollusca
done
clear
B)
Nematoda
done
clear
C)
Porifera
done
clear
D)
Chordata
done
clear
View Answer play_arrow
question_answer 141) The percentage of mutant alleles, which are produced spontaneously and is beneficial to the organism is about
A)
0.1
done
clear
B)
10
done
clear
C)
50
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 142) Isotonic solutions have same
A)
normality
done
clear
B)
pH
done
clear
C)
temperature
done
clear
D)
osmolarity
done
clear
View Answer play_arrow
question_answer 143) These organisms have chloride cells
A)
plants
done
clear
B)
freshwater fish -
done
clear
C)
Protozoa
done
clear
D)
birds
done
clear
View Answer play_arrow
question_answer 144) The major nitrogenous waste of shark is
A)
urea
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
uric acid
done
clear
D)
amino acid
done
clear
View Answer play_arrow
question_answer 145) Metanephridia is the excretory system in
A)
cockroach
done
clear
B)
locust
done
clear
C)
earthworm
done
clear
D)
birds
done
clear
View Answer play_arrow
question_answer 146) Angiotensin II works as
A)
enzyme
done
clear
B)
antibody
done
clear
C)
coenzyme
done
clear
D)
hormone
done
clear
View Answer play_arrow
question_answer 147) Actih is
A)
an enzyme
done
clear
B)
a contractile protein
done
clear
C)
a hormone
done
clear
D)
a vitamin
done
clear
View Answer play_arrow
question_answer 148) Interferons are
A)
nucleoproteins
done
clear
B)
glycoproteins
done
clear
C)
lipoproteins
done
clear
D)
ribosomal proteins
done
clear
View Answer play_arrow
question_answer 149) This is a primary lymphoid organ
A)
thymus
done
clear
B)
lymph nodes
done
clear
C)
spleen
done
clear
D)
tonsils
done
clear
View Answer play_arrow
question_answer 150) Matching of the following is necessary for successful organ transplant
A)
Rh factor
done
clear
B)
ABO blood group
done
clear
C)
MHC antigens
done
clear
D)
Hypersensitivity
done
clear
View Answer play_arrow