question_answer 1) An electron makes transition from\[n=3\]to the orbit \[n=2\]of a hydrogen atom (ionization potential 13.6 eV). The energy of the photon emitted in the process is
A)
1.89 eV
done
clear
B)
2.55 eV
done
clear
C)
12.09 eV
done
clear
D)
12.75 eV
done
clear
View Answer play_arrow
question_answer 2) The moment of inertia of a uniform thin rod of length L and mass M about an axis passing through a point at a distance of \[\frac{L}{3}\]from one of its ends and perpendicular to the rod is
A)
\[\frac{7M{{L}^{2}}}{48}\]
done
clear
B)
\[\frac{M{{L}^{2}}}{9}\]
done
clear
C)
\[\frac{M{{L}^{2}}}{12}\]
done
clear
D)
\[\frac{M{{L}^{2}}}{3}\]
done
clear
View Answer play_arrow
question_answer 3) Two satellites A and B go around a planet P in circular orbits having radii 4R and R respectively. If the speed of the satellite A is 3v, the speed of the satellite B will be
A)
12v
done
clear
B)
6v
done
clear
C)
\[\frac{4}{3}v\]
done
clear
D)
\[\frac{3}{2}v\]
done
clear
View Answer play_arrow
question_answer 4) A closed vessel contains 8 g of oxygen and 7g of nitrogen. The total pressure is 10 atm at a given temperature. If now oxygen is absorbed by introducing a suitable absorbent the pressure of the remaining gas (in atm) will be
A)
2
done
clear
B)
10
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 5) A thin square steel plate with each side equal to 10 cm is heated by a blacksmith. The rate radiated energy by the heated plate is 1134W. The temperature of the hot steel plate is (Stefan's constant\[\sigma =5.67\times {{10}^{-8}}\]\[W\,{{m}^{-2}}{{K}^{-4}},\]emissivity of the plate = 1)
A)
1000 K
done
clear
B)
1189 K
done
clear
C)
2000 K
done
clear
D)
2378 K
done
clear
View Answer play_arrow
question_answer 6) The time period of a simple pendulum of length L as measured in an elevator descending with acceleration\[\frac{g}{3}\]is
A)
\[2\pi \sqrt{\frac{3L}{2g}}\]
done
clear
B)
\[\pi \sqrt{\frac{3L}{g}}\]
done
clear
C)
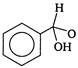
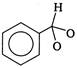
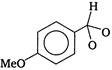
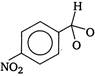
\[2\pi \sqrt{\left( \frac{3L}{g} \right)}\]
done
clear
D)
\[2\pi \sqrt{\frac{2L}{g}}\]
done
clear
View Answer play_arrow
question_answer 7) A charged particle is free to move in an electric field. It will travel
A)
always along a line of force
done
clear
B)
along a line of force, if its initial velocity is zero
done
clear
C)
along a line of force, if it has same initial velocity in the direction of an active angle with the line of force
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 8) A charged particle of mass m and charge q is released from rest in uniform electric field E. Neglecting the effect of gravity, the kinetic energy of the charged particle after t second is
A)
\[\frac{E{{q}^{2}}M}{2{{t}^{2}}}\]
done
clear
B)
\[\frac{2{{E}^{2}}{{t}^{2}}}{mq}\]
done
clear
C)
\[\frac{{{E}^{2}}{{q}^{2}}{{t}^{2}}}{2m}\]
done
clear
D)
\[\frac{Eqt}{t}\]
done
clear
View Answer play_arrow
question_answer 9) The capacitance of a parallel plate capacitor with air as medium is\[3\mu F\]. With the introduction of a dielectric medium between the plates, the capacitance becomes\[15\mu F\]. The permittivity of the medium is
A)
\[5{{C}^{2}}{{N}^{-1}}{{m}^{-2}}\]
done
clear
B)
\[15{{C}^{2}}{{N}^{-1}}{{m}^{-2}}\]
done
clear
C)
\[0.44\times {{10}^{-10}}{{C}^{2}}{{N}^{-1}}{{m}^{-2}}\]
done
clear
D)
\[8.845\times {{10}^{-11}}{{C}^{2}}{{N}^{-1}}{{m}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 10) The magnetic moment produced in a substance of 1 g is\[6\times {{10}^{-7}}A-{{m}^{2}}\]. If its density is\[5g/c{{m}^{3}},\]then the intensity of magnetization in A/m will be
A)
\[8.3\times {{10}^{6}}\]
done
clear
B)
3.0
done
clear
C)
\[1.2\times {{10}^{-7}}\]
done
clear
D)
\[3\times {{10}^{-6}}\]
done
clear
View Answer play_arrow
question_answer 11) Mark the wrong statement.
A)
Sliding of molecular layer is much easier than compression or expression
done
clear
B)
Reciprocal of bulk modulus of elasticity is called compressibility
done
clear
C)
It is difficult to twist a long rod as compared to small rod
done
clear
D)
Hollow shaft is much stronger than a solid rod of same mass
done
clear
View Answer play_arrow
question_answer 12) A body moves along a circular path of radius 10m and the coefficient of friction is 0.5. What should be its angular speed in rad/s, if it does not slip from the surface?
A)
5
done
clear
B)
10
done
clear
C)
0.1
done
clear
D)
0.7
done
clear
View Answer play_arrow
question_answer 13) A stone is projected from the ground with velocity 50 m/s at an angle of\[30{}^\circ \]. It crosses a wall after 3 s. How far beyond the wall the stone will strike the ground?\[(g=10\text{ }m/{{s}^{2}})\]
A)
90.2m
done
clear
B)
89.6m
done
clear
C)
86.6m
done
clear
D)
70.2m
done
clear
View Answer play_arrow
question_answer 14) A body of mass M at rest explodes into three pieces, two of which of mass\[\frac{M}{4}\]each are thrown off in perpendicular directions with velocities of 3 m/s and 4 m/s respectively. The third piece will be thrown off with velocity of
A)
1.5 m/s
done
clear
B)
2.0 m/s
done
clear
C)
2.5 m/s
done
clear
D)
3.0 m/s
done
clear
View Answer play_arrow
question_answer 15) Two identical blocks A and B, each of mass m resting on smooth floor are connected by a light spring of natural length L and spring constant k with the spring at its natural length. A third identical block C (mass m) moving with a speed v along the line joining A and B collides with A. The maximum compression in the spring is
A)
\[v\sqrt{\frac{m}{2k}}\]
done
clear
B)
\[m\sqrt{\frac{v}{2k}}\]
done
clear
C)
\[\sqrt{\frac{mv}{k}}\]
done
clear
D)
\[\frac{mv}{2k}\]
done
clear
View Answer play_arrow
question_answer 16) A\[500\mu F\]capacitor is charged at the steady rate of\[100\mu C/s\]. How long will it take to raise the potential difference between the plates of the capacitor to 10 V?
A)
5s
done
clear
B)
10s
done
clear
C)
50s
done
clear
D)
100s
done
clear
View Answer play_arrow
question_answer 17) An\[8\mu F\]capacitor is connected across 220 V, 50 Hz line. What is the peak value of the charge through capacitor?
A)
\[2.5\times {{10}^{-3}}C\]
done
clear
B)
\[2.5\times {{10}^{-4}}C\]
done
clear
C)
\[5\times {{10}^{-5}}C\]
done
clear
D)
\[7.5\times {{10}^{-2}}C\]
done
clear
View Answer play_arrow
question_answer 18) A hydrogen-like atom emits radiations of frequency\[2.7\times {{10}^{15}}Hz\]when it makes a transition from\[n=2\]to\[n=1\]. The frequency emitted in a transition from\[n=3\]to\[n=1\]will be
A)
\[1.8\times {{10}^{15}}Hz\]
done
clear
B)
\[3.2\times {{10}^{15}}Hz\]
done
clear
C)
\[4.7\times {{10}^{5}}Hz\]
done
clear
D)
\[6.9\times {{10}^{15}}Hz\]
done
clear
View Answer play_arrow
question_answer 19) The minimum energy to ionize an atom is the energy required to
A)
add one electron to the atom
done
clear
B)
excite the atom from its ground state to its first excited state
done
clear
C)
remove one outermost electron from the atom
done
clear
D)
remove one innermost electron from the atom
done
clear
View Answer play_arrow
question_answer 20) When 1 cm thick surface is illuminated with light of wavelength X, the stopping potential is V. When the same surface is illuminated by light of wavelength 2X, the stopping potential is V/3. Threshold wavelength for metallic surface is
A)
\[4\lambda /3\]
done
clear
B)
\[4\lambda\]
done
clear
C)
\[6\lambda\]
done
clear
D)
\[8\lambda /3\]
done
clear
View Answer play_arrow
question_answer 21) The human eye can barely detect a yellow light (6000A) that delivers \[1.7\times {{10}^{-18}}W\] to the retina. Nearly how many photons per second does the retina receive?
A)
50
done
clear
B)
5
done
clear
C)
500
done
clear
D)
More than 5 million
done
clear
View Answer play_arrow
question_answer 22) An object is placed 21 cm in front of a concave mirror of radius of curvature 10cm. A glass slab of thickness 3 cm and \[\mu =1.5\] is then placed close to the mirror in the space between the object and the mirror. The position of final image formed is
A)
\[-3.94\text{ }cm\]
done
clear
B)
4.3 cm
done
clear
C)
\[-4.93cm\]
done
clear
D)
3.94cm
done
clear
View Answer play_arrow
question_answer 23) A convex lens of focal length 1.0 m and a concave lens of focal length 0.25 m are 0.75m apart. A parallel beam of light is incident in the convex lens. The beam emerging after refraction from both lenses is
A)
parallel to principal axis
done
clear
B)
convergent
done
clear
C)
divergent
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 24) The radii of curvature of the two surfaces of a lens are 20 cm and 30 cm and the refractive index of the material of the lens is 1.5. If the lens is concavo-convex, then the focal length of the lens is
A)
24cm
done
clear
B)
10cm
done
clear
C)
15cm
done
clear
D)
120cm
done
clear
View Answer play_arrow
question_answer 25) Two instruments having stretched strings are being played in unison. When the tension in one of the instruments is increased by 1%, 3 beats are produced in 2 s. The initial frequency of vibration of each wire is
A)
600 Hz
done
clear
B)
300 Hz
done
clear
C)
200 Hz
done
clear
D)
150 Hz
done
clear
View Answer play_arrow
question_answer 26) Two conducting spheres of radii \[{{R}_{1}}\] and \[{{R}_{2}}\] are charged with charges \[{{Q}_{1}}\] and \[{{Q}_{2}}\] respectively. On bringing them in contact, there is
A)
no change in the energy of the system
done
clear
B)
an increase in the energy of the system if \[{{Q}_{1}}{{R}_{2}}\ne {{Q}_{2}}{{R}_{1}}\]
done
clear
C)
always a decrease in the energy of the system
done
clear
D)
a decrease in the energy of the system if \[{{Q}_{1}}{{R}_{2}}={{Q}_{2}}{{R}_{1}}\]
done
clear
View Answer play_arrow
question_answer 27) An electron moving with the speed \[5\times {{10}^{6}}\] per sec is shooted parallel to the electric field of intensity \[1\times 10s\text{ }N/C\] . Field is responsible for the retardation of motion of electron. Now evaluate the distance travelled by the electron before coming to rest for an instant (mass of \[e=9\times {{10}^{-31}}kg,\] charge \[=1.6\times {{10}^{-19}}C\] )
A)
7 m
done
clear
B)
0.7 mm
done
clear
C)
7cm
done
clear
D)
0.7cm
done
clear
View Answer play_arrow
question_answer 28)
A source of sound is travelling at \[\frac{100}{3}m{{s}^{-1}}\] along a road, towards a point A. When the source is 2 m away from A, a person standing at a point 0 on a road perpendicular of
A)
620 Hz
done
clear
B)
680 Hz
done
clear
C)
720 Hz
done
clear
D)
840 Hz
done
clear
View Answer play_arrow
question_answer 29) The sun delivers \[104\text{ }W/{{m}^{2}}\] of electromagnetic flux to the earth's surface. The total power that is incident on a roof of dimensions \[(10\times 10)\text{ }{{m}^{2}}\] will be
A)
104 W
done
clear
B)
105 W
done
clear
C)
106 W
done
clear
D)
107 W
done
clear
View Answer play_arrow
question_answer 30) X-rays are not used for radar purposes, because they are not
A)
reflected by target
done
clear
B)
partly absorbed by target
done
clear
C)
electromagnetic waves
done
clear
D)
completely absorbed by target
done
clear
View Answer play_arrow
question_answer 31) A 50 Hz AC current of crest value 1 A flows through the primary coil of a transformer. If mutual induction between primary and secondary is 1.5 H, the crest voltage induced in the secondary coil is
A)
272V
done
clear
B)
320V
done
clear
C)
415V
done
clear
D)
471V
done
clear
View Answer play_arrow
question_answer 32) Find the inductance L of a solenoid of length I whose windings are made of material of density D and resistivity p. The winding resistance is R.
A)
\[\frac{{{\mu }_{0}}}{4\pi l}.\frac{Rm}{\rho D}\]
done
clear
B)
\[\frac{{{\mu }_{0}}}{4\pi R}.\frac{lm}{\rho D}\]
done
clear
C)
\[\frac{{{\mu }_{0}}}{4\pi l}.\frac{{{R}^{2}}m}{\rho D}\]
done
clear
D)
\[\frac{{{\mu }_{0}}}{2\pi R}.\frac{lm}{\rho D}\]
done
clear
View Answer play_arrow
question_answer 33) A toroid is along coil of wire, wound over a circular core. The coefficient of self-inductance of the toroid is given by (radius = r), when the magnetic field is within it is uniform and\[R>>r\]
A)
\[L=\frac{{{\mu }_{0}}N{{r}^{2}}}{2R}\]
done
clear
B)
\[L=\frac{{{\mu }_{0}}Nr}{2R}\]
done
clear
C)
\[L=\frac{{{\mu }_{0}}N{{r}^{2}}}{R}\]
done
clear
D)
\[L=\frac{{{\mu }_{0}}{{N}^{2}}{{r}^{2}}}{2R}\]
done
clear
View Answer play_arrow
question_answer 34) A galvanometer of resistance\[50\,\Omega \]is connected to a battery of 3 V along with a resistance of\[2950\,\Omega \]in series. A full scale deflection of 30 divisions is obtained in the galvanometer. In order to reduce this deflection to 20 divisions the resistance in series should be
A)
\[4450\,\Omega \]
done
clear
B)
\[5050\,\Omega \]
done
clear
C)
\[5550\,\Omega \]
done
clear
D)
\[6050\,\Omega \]
done
clear
View Answer play_arrow
question_answer 35) A magnet is suspended in such a way that it oscillates in the horizontal plane. It makes 20 oscillations per minute at a plane where dip angle is\[30{}^\circ \]and 15 oscillations per minute at a place where dip angle is\[60{}^\circ \]. The ratio of earth's magnetic fields at two places is
A)
\[3\sqrt{3}:8\]
done
clear
B)
\[16:9\sqrt{3}\]
done
clear
C)
\[4:9\]
done
clear
D)
\[2\sqrt{2}:3\]
done
clear
View Answer play_arrow
question_answer 36) A wire carrying current i and other carrying 2i in the same direction produce a magnetic field B at the midpoint. What will be the field when 2i current is switched off?
A)
B/2
done
clear
B)
2B
done
clear
C)
B
done
clear
D)
4B
done
clear
View Answer play_arrow
question_answer 37) An electric immersion heater of 1.08 kW is immersed in water. After the water has reached a temperature of\[100{}^\circ C,\]how much time will be required to produce 100 g of steam?
A)
50s
done
clear
B)
420s
done
clear
C)
105s
done
clear
D)
210s
done
clear
View Answer play_arrow
question_answer 38) The resistance of the filament of a lamp increases with the increase in temperature. A lamp rated 100 W and 200 V is connected across 220 V power supply. If the voltage drops by 10%, then the power of the lamp will be
A)
90 W
done
clear
B)
81 W
done
clear
C)
between 90 and 100W
done
clear
D)
between 81 and 90W
done
clear
View Answer play_arrow
question_answer 39) A parallel plate air capacitor of capacitance\[{{C}_{0}}\]is connected to a cell of emf V and then disconnected from it. A dielectric slab of dielectric constant K, which can just fill the air gap of the capacitor, is now inserted in it. Which of the following is incorrect?
A)
The potential difference between the plates decreases K times
done
clear
B)
The energy stored in the capacitor decreases K times
done
clear
C)
The change in energy\[\frac{1}{2}{{C}_{0}}{{V}^{2}}(K-1)\]
done
clear
D)
The change in energy\[\frac{1}{2}{{C}_{0}}{{V}^{2}}\left( \frac{1}{K}-1 \right)\]
done
clear
View Answer play_arrow
question_answer 40) A body of mass 6 kg is under a force which causes displacement in it given by\[s=\frac{{{t}^{2}}}{4}m\]where t is time. The work done by the force in 2 sis
A)
12 J
done
clear
B)
9J
done
clear
C)
6J
done
clear
D)
3J
done
clear
View Answer play_arrow
question_answer 41) The Reynolds number of a flow is the ratio of
A)
gravity to viscous force
done
clear
B)
gravity force to pressure force
done
clear
C)
inertia force to viscous force
done
clear
D)
viscous force to pressure force
done
clear
View Answer play_arrow
question_answer 42) The kinetic energy of 1 g molecule of a gas at normal temperature and pressure is\[(R=8.31\text{ }J/mol-K)\]
A)
\[1.3\times {{10}^{2}}J\]
done
clear
B)
\[2.7\times {{10}^{2}}J\]
done
clear
C)
\[0.56\times {{10}^{4}}J\]
done
clear
D)
\[3.4\times {{10}^{3}}J\]
done
clear
View Answer play_arrow
question_answer 43) An ideal gas heat engine operates in Carnot cycle between\[227{}^\circ C\]and\[127{}^\circ C\]. It absorbs \[6\times {{10}^{4}}\]cal of heat at higher temperature. Amount of heat converted to work is
A)
\[2.4\times {{10}^{4}}\text{ }cal\]
done
clear
B)
\[6\times {{10}^{4}}\text{ }cal\]
done
clear
C)
\[1.2\times {{10}^{4}}cal\]
done
clear
D)
\[4.8\times {{10}^{4}}\text{ }cal\]
done
clear
View Answer play_arrow
question_answer 44) A charged particle enters a magnetic field H with its initial velocity making an angle of\[45{}^\circ \] with H. The path of the particle will be
A)
straight line
done
clear
B)
a circle
done
clear
C)
an ellipse
done
clear
D)
a helix
done
clear
View Answer play_arrow
question_answer 45) The dimensional formula for Boltzmann's constant is
A)
\[[M{{L}^{2}}{{T}^{-2}}{{\theta }^{-1}}]\]
done
clear
B)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
C)
\[[M{{L}^{o}}{{T}^{-2}}{{\theta }^{-2}}]\]
done
clear
D)
\[[M{{L}^{-2}}{{T}^{-1}}{{\theta }^{-1}}]\]
done
clear
View Answer play_arrow
question_answer 46) A particle moves along a straight line OX. At a time t (in second) the distance\[x\](in metre) of the particle from 0 is given by \[x=40+12t-{{t}^{3}}\]. How long would the particle travel before coming to rest?
A)
24m
done
clear
B)
40m
done
clear
C)
56m
done
clear
D)
16m
done
clear
View Answer play_arrow
question_answer 47) The velocity of an electron in the second orbit of sodium atom (atomic number = 11) is v. The velocity of an electron in its fifth orbit will be
A)
\[v\]
done
clear
B)
\[\frac{22}{5}v\]
done
clear
C)
\[\frac{5}{2}v\]
done
clear
D)
\[\frac{2}{5}v\]
done
clear
View Answer play_arrow
question_answer 48) Choose the correct statement.
A)
When we heat a semiconductor its resistance increases
done
clear
B)
When we heat a semiconductor its resistance decreases
done
clear
C)
When we cool a semiconductor to 0 K then it becomes superconductor
done
clear
D)
Resistance of a semiconductor is independent of temperature
done
clear
View Answer play_arrow
question_answer 49) 3.8 days is the half-life period of a sample. After how many days; the sample will become\[\frac{1}{8}\]th of the original substance?
A)
11.4
done
clear
B)
3.8
done
clear
C)
3
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 50) If\[E=at+b{{t}^{2}},\]what is the neutral temperature?
A)
\[-\frac{a}{2b}\]
done
clear
B)
\[+\frac{a}{2b}\]
done
clear
C)
\[-\frac{a}{b}\]
done
clear
D)
\[+\frac{a}{b}\]
done
clear
View Answer play_arrow
question_answer 51)
The IUPAC name of
A)
3-iso-propylheptane
done
clear
B)
3-iso-propylpentane
done
clear
C)
2-methyl-3-propylhexane
done
clear
D)
4-isoi-propyl heptane
done
clear
View Answer play_arrow
question_answer 52) Which of the following molecules fails to exhibit optical activity?
A)
\[C{{H}_{2}}Cl-CHDBr\]
done
clear
B)
\[C{{H}_{3}}CHC{{l}_{2}}\]
done
clear
C)
\[C{{H}_{3}}CHDCl\]
done
clear
D)
\[C{{H}_{2}}H-CHClC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 53) In the following reaction sequence, X is\[X\xrightarrow{Bro\min ation}Y\xrightarrow{NaN{{O}_{2}}/HCl}\]\[Z\xrightarrow[{{C}_{2}}{{H}_{5}}OH]{Boiling}Tribromobenzene\]
A)
benzoic acid
done
clear
B)
salicylic acid
done
clear
C)
phenol
done
clear
D)
aniline
done
clear
View Answer play_arrow
question_answer 54) \[K{{O}_{2}}+S\xrightarrow{\Delta }(A)\xrightarrow{BaC{{l}_{2}}}\underset{white\text{ }ppt}{\mathop{(B)}}\,\]\[\xrightarrow[\Delta ]{Carbon}(C)\xrightarrow{HCl}\underset{gas}{\mathop{(D)}}\,\]The correct statement is
A)
B is\[BaS{{O}_{4}}\]and D is\[{{H}_{2}}S\]
done
clear
B)
A is\[BaS\]and D is\[S{{O}_{2}}\]
done
clear
C)
C is\[BaS\]and D is\[S{{O}_{2}}\]
done
clear
D)
A is\[{{K}_{2}}S{{O}_{3}}\]and B is\[BaS\]
done
clear
View Answer play_arrow
question_answer 55) In the reaction,\[4HN{{O}_{3}}+{{P}_{4}}{{\text{O}}_{10}}\xrightarrow{{}}4HP{{O}_{3}}+X,\]the product X, is
A)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
B)
\[{{N}_{2}}{{O}_{3}}\]
done
clear
C)
\[N{{O}_{2}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 56) Nitrolim is a mixture of
A)
\[Ca{{(CN)}_{2}}+graphite\]
done
clear
B)
\[Ca{{C}_{2}}+graphite\]
done
clear
C)
\[CaC{{N}_{2}}+graphite\]
done
clear
D)
\[CaC{{N}_{2}}+{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 57) Which of the following aqueous solutions has minimum freezing point?
A)
\[0.01\,m\,NaCl\]
done
clear
B)
\[0.005{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[0.005\text{ }m\text{ }Mg{{l}_{2}}\]
done
clear
D)
\[0.005\text{ }m\text{ }MgS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 58) The STP volume of oxygen liberated by 2 A of current when passed through acidulated water for 3 min and 13 s is
A)
120 cc
done
clear
B)
22.4 cc
done
clear
C)
11.2cc
done
clear
D)
44.8 cc
done
clear
View Answer play_arrow
question_answer 59) \[{{K}_{p}}\]of a reaction at 300 K is 6 atm and 2 atm at 450 K. Which of the statements is incorrect about this reaction, if\[\Delta {{n}_{g}}=1\]?
A)
The reaction is exothermic
done
clear
B)
The rate of backward reaction increases more than that of forward reaction with increase in temperature
done
clear
C)
\[{{E}_{a}}\]for the forward reaction is more than that of backward reaction
done
clear
D)
The difference between heat of reaction at constant pressure and that at constant volume is RT
done
clear
View Answer play_arrow
question_answer 60) In alkaline medium, KMn04 reacts as follows\[2KMn{{O}_{4}}+2KOH\xrightarrow[{}]{{}}2{{K}_{2}}Mn{{O}_{4}}\] \[+{{H}_{2}}O+[O]\] Therefore, the equivalent weight of\[KMn{{O}_{4}}\]will be
A)
31.6
done
clear
B)
52.7
done
clear
C)
7.0
done
clear
D)
158.0
done
clear
View Answer play_arrow
question_answer 61) When\[_{92}{{U}^{235}}\]loses one a-particle, the new element will belong to group
A)
\[IB\]
done
clear
B)
\[IA\]
done
clear
C)
\[IIIB\]
done
clear
D)
\[VB\]
done
clear
View Answer play_arrow
question_answer 62) The correct order of bond angles is
A)
\[NO_{2}^{-}>NO_{2}^{+}>N{{O}_{2}}\]
done
clear
B)
\[NO_{2}^{+}>NO_{2}^{-}>N{{O}_{2}}\]
done
clear
C)
\[N{{O}_{2}}>NO_{2}^{+}>NO_{2}^{-}\]
done
clear
D)
\[NO_{2}^{+}>N{{O}_{2}}>NO_{2}^{-}\]
done
clear
View Answer play_arrow
question_answer 63) Cod liver oil is
A)
fat dispersed in water
done
clear
B)
water dispersed in fat
done
clear
C)
water dispersed in oil
done
clear
D)
fat dispersed in fat
done
clear
View Answer play_arrow
question_answer 64) Cassiterite is concentrated by
A)
levigation
done
clear
B)
electromagnetic separation
done
clear
C)
floatation
done
clear
D)
liquefaction
done
clear
View Answer play_arrow
question_answer 65) The stability of the hydrides follows the order
A)
\[N{{H}_{3}}>P{{H}_{3}}>As{{H}_{3}}>Sb{{H}_{3}}\]
done
clear
B)
\[N{{N}_{3}}<P{{H}_{3}}<Sb{{H}_{3}}<As{{H}_{3}}\]
done
clear
C)
\[P{{H}_{3}}>N{{H}_{3}}>As{{H}_{3}}>Sb{{H}_{3}}\]
done
clear
D)
\[As{{H}_{3}}>N{{H}_{3}}>P{{H}_{3}}>Sb{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 66) Catalytic activity of transition metals depends on
A)
their ability to exist in different oxidation states
done
clear
B)
the size of the metal atoms
done
clear
C)
the number of empty atomic orbitals available
done
clear
D)
All of the above are correct
done
clear
View Answer play_arrow
question_answer 67) Which of the following complex species does not involve\[{{d}^{2}}s{{p}^{3}}\]hybridisation?
A)
\[{{[Co{{F}_{6}}]}^{3-}}\]
done
clear
B)
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
C)
\[{{[Fe{{(CN)}_{6}}]}^{3-}}\]
done
clear
D)
\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 68) In the complex,\[PtC{{l}_{4}}.3N{{H}_{3}},\]the number of ionisable chlorines is
A)
four
done
clear
B)
two
done
clear
C)
one
done
clear
D)
three
done
clear
View Answer play_arrow
question_answer 69) Which of the following has highest chlorine content?
A)
Pyrene
done
clear
B)
DDT
done
clear
C)
Chloral
done
clear
D)
Gammaxane
done
clear
View Answer play_arrow
question_answer 70) Benzene on oxidation with acidified \[N{{a}_{2}}C{{r}_{2}}{{O}_{7}}\]gives
A)
benzyl alcohol
done
clear
B)
benzaldehyde
done
clear
C)
benzoic acid
done
clear
D)
No reaction occurs
done
clear
View Answer play_arrow
question_answer 71) The correct order of increasing basicity of the following is\[{{H}_{2}}O,O{{H}^{-}},\text{ }C{{H}_{3}}OH,\text{ }C{{H}_{3}}{{O}^{-}}\]
A)
\[C{{H}_{3}}OH<{{H}_{2}}O~<O{{H}^{-}}~<C{{H}_{3}}{{O}^{-}}\]
done
clear
B)
\[{{H}_{2}}O~<C{{H}_{3}}OH<C{{H}_{3}}{{O}^{-}}<O{{H}^{-}}\]
done
clear
C)
\[{{H}_{2}}O~<C{{H}_{3}}OH<O{{H}^{-}}<C{{H}_{3}}{{O}^{-}}\]
done
clear
D)
\[C{{H}_{3}}OH<{{H}_{2}}O~<C{{H}_{3}}{{O}^{-}}<O{{H}^{-}}\]
done
clear
View Answer play_arrow
question_answer 72) \[A\xrightarrow[{{H}_{2}}S{{O}_{4}}]{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}B\xrightarrow[\begin{smallmatrix} Vigorous \\ oxidation \end{smallmatrix}]{[O]}C{{H}_{3}}COOH\]If B in the given sequence is propanone then A is
A)
ethyl alcohol
done
clear
B)
iso-propyl alcohol
done
clear
C)
propyl alcohol
done
clear
D)
tert-pentyl alcohol
done
clear
View Answer play_arrow
question_answer 73) In a Cannizaro reaction, the intermediate that will be best hydride donor is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 74) Aniline on heating with cone\[HN{{O}_{3}}+\]cone\[{{H}_{2}}S{{O}_{4}}\]mixture yields
A)
o- and p-nitroanilines
done
clear
B)
m-nitroaniline
done
clear
C)
a black tarry matter
done
clear
D)
no reaction
done
clear
View Answer play_arrow
question_answer 75) In which of the following reaction, pressure has no effect on equilibrium?
A)
\[{{N}_{2}}{{O}_{4}}(g)2N{{O}_{2}}(g)\]
done
clear
B)
\[2S{{O}_{2}}(g)+{{O}_{2}}(g)2S{{O}_{3}}(g)\]
done
clear
C)
\[C{{O}_{2}}(g)+{{H}_{2}}(g)CO(g)+{{H}_{2}}O(g)\]
done
clear
D)
\[{{N}_{2}}(g)+3{{H}_{2}}(g)2N{{H}_{3}}(g)\]
done
clear
View Answer play_arrow
question_answer 76) Two moles of HI were heated in a sealed tube at\[440{}^\circ C\]till the equilibrium was reached.\[HI\] was found to be 22% decomposed. The equilibrium constant for dissociation is
A)
0.282
done
clear
B)
0.0796
done
clear
C)
0.0199
done
clear
D)
1.99
done
clear
View Answer play_arrow
question_answer 77) Assertion Most probable velocity is the velocity possessed by maximum fraction of molecules at the same temperature. Reason (R) on collision, more and more molecules acquire higher speed at the same temperature.
A)
Both Assertion and Reason are true and the Reason is the true explanation of the Assertion.
done
clear
B)
Both Assertion and Reason are true, but Reason is not the true explanation of Assertion.
done
clear
C)
Assertion is true, but Reason is false.
done
clear
D)
Assertion is false, but Reason is true.
done
clear
View Answer play_arrow
question_answer 78) The first emission line in the atomic spectrum of hydrogen in the Balmer series appears at
A)
\[\frac{9R}{400}c{{m}^{-1}}\]
done
clear
B)
\[\frac{7R}{144}c{{m}^{-1}}\]
done
clear
C)
\[\frac{3R}{4}c{{m}^{-1}}\]
done
clear
D)
\[\frac{5R}{36}c{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 79) If for\[{{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}{{H}_{2}}O(g),\]\[\Delta {{H}_{1}}\]is the enthalpy of reaction and fo\[{{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow[{}]{{}}{{H}_{2}}O(l)\]\[\Delta {{H}_{2}}\]is enthalpy of reaction, then
A)
\[\Delta {{H}_{1}}>\Delta {{H}_{2}}\]
done
clear
B)
\[\Delta {{H}_{1}}=\Delta {{H}_{2}}\]
done
clear
C)
\[\Delta {{H}_{1}}<\Delta {{H}_{2}}\]
done
clear
D)
\[\Delta {{H}_{1}}+\Delta {{H}_{2}}=0\]
done
clear
View Answer play_arrow
question_answer 80) A sodium cation has a different number of electrons from
A)
\[{{O}^{2-}}\]
done
clear
B)
\[{{F}^{-}}\]
done
clear
C)
\[L{{i}^{+}}\]
done
clear
D)
\[A{{l}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 81) Oxidation of thiosulphate ion by iodine gives
A)
\[SO_{3}^{2-}\]
done
clear
B)
\[SO_{4}^{2-}\]
done
clear
C)
\[{{S}_{4}}O_{6}^{2-}\]
done
clear
D)
\[{{S}_{2}}O_{5}^{2-}\]
done
clear
View Answer play_arrow
question_answer 82) Which of the following bonds has the lusher energy?
A)
\[SeSe\]
done
clear
B)
\[TeTe\]
done
clear
C)
\[SS\]
done
clear
D)
\[OO\]
done
clear
View Answer play_arrow
question_answer 83) Number of \[\pi -\] electrons in cyclobutadienyl anlon\[{{({{C}_{4}}{{H}_{4}})}^{2-}}\]is
A)
2
done
clear
B)
4
done
clear
C)
6
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 84) Cetane number of diesel fuel will increase with the addition of
A)
n-decane
done
clear
B)
n-hexadecane
done
clear
C)
n-hexane
done
clear
D)
a-methyihaphthalene
done
clear
View Answer play_arrow
question_answer 85) lodoform can be obtained on warming\[NaOH\] and iodine with
A)
\[C{{H}_{3}}C{{H}_{2}}CH(OH)C{{H}_{3}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}CH\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,{{C}_{2}}{{H}_{5}}\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-OC{{H}_{3}}\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}CHC{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 86) Which is an example of thermosetting polymer?
A)
Polythene
done
clear
B)
PVC
done
clear
C)
Neoprene
done
clear
D)
Bakelite
done
clear
View Answer play_arrow
question_answer 87) Analysis shows that nickel oxide has the formula\[N{{i}_{0.98}}O\]. What fraction of the nickel exists as\[N{{i}^{3+}}\]ions?
A)
1 %
done
clear
B)
2 %
done
clear
C)
4%
done
clear
D)
8%
done
clear
View Answer play_arrow
question_answer 88) The molecule/ion which has pyramidal shape is:
A)
\[PC{{l}_{3}}\]
done
clear
B)
\[S{{O}_{3}}\]
done
clear
C)
\[CO_{3}^{2-}\]
done
clear
D)
\[NO_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 89) By Pajan's rule covalent character increases with increase in size of the anion. Identify the correct order of melting point of calcium.
A)
\[Ca{{F}_{2}}<CaC{{l}_{2}}<CaB{{r}_{2}}<Ca{{I}_{2}}\]
done
clear
B)
\[CaC{{l}_{2}}<Ca{{F}_{2}}<CaB{{r}_{2}}<Ca{{I}_{2}}\]
done
clear
C)
\[Ca{{F}_{2}}>CaC{{l}_{2}}>CaB{{r}_{2}}>Ca{{I}_{2}}\]
done
clear
D)
\[CaC{{l}_{2}}>Ca{{F}_{2}}>CaB{{r}_{2}}>Ca{{I}_{2}}\]
done
clear
View Answer play_arrow
question_answer 90) Considering\[NO,\text{ }N{{O}^{+}}\]and\[N{{O}^{-}}\]we say that
A)
among the three, the one isoelectronic with\[{{N}_{2}}\]alone is diamagnetic
done
clear
B)
among the three, the one isoelectronic with\[{{O}_{2}}\]alone is paramagnetic
done
clear
C)
all the three species are paramagnetic
done
clear
D)
the three overlaps are stronger than\[{{N}_{2}}\]or \[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 91)
For a reaction\[A+2B\xrightarrow{{}}C+D\]the following data were obtain: Expt. Initial concentration \[(mol\,{{L}^{-1}})\] Initial rate of formation of D \[(mol\,{{L}^{-1}}{{\min }^{-1}})\] [A] [B] 1. 0.1 0.1 \[6.0\times {{10}^{-3}}\] 2. 0.3 0.2 \[7.2\times {{10}^{-2}}\] 3. 0.3 0.1 \[2.88\times {{10}^{-1}}\] 4. 0.4 0.1 \[2.4\times {{10}^{-2}}\]
The correct rate law expression will be
A)
\[Rate=k[A][B]\]
done
clear
B)
\[Rate=k[A]{{[B]}^{2}}\]
done
clear
C)
\[Rate=k{{[A]}^{2}}{{[B]}^{2}}\]
done
clear
D)
\[Rate=k{{[A]}^{2}}[B]\]
done
clear
View Answer play_arrow
question_answer 92) The standard reduction potential of three metals\[X,Y\]and \[Z\]are 0.52, -3.03 and -1.18V respectively. The order or reducing power of the corresponding metals is
A)
\[Y>Z>X\]
done
clear
B)
\[X>Y>Z\]
done
clear
C)
\[Z>Y>X\]
done
clear
D)
\[Z>X>Y\]
done
clear
View Answer play_arrow
question_answer 93) Which of the following is not a redox rection?
A)
\[CaC{{O}_{3}}\xrightarrow{{}}CaO+C{{O}_{2}}\]
done
clear
B)
\[{{O}_{2}}+2{{H}_{2}}\xrightarrow[{}]{{}}2{{H}_{2}}O\]
done
clear
C)
\[Na+{{H}_{2}}O\xrightarrow[{}]{{}}NaOH+\frac{1}{2}{{H}_{2}}\]
done
clear
D)
\[MnC{{l}_{3}}\xrightarrow{{}}MnC{{l}_{2}}+\frac{1}{2}C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 94) In which of the following pair, the EAN of central metal atom is not same?
A)
\[{{[Fe{{F}_{6}}]}^{3-}}\]and\[{{[Fe{{(CN)}_{6}}]}^{3-}}\]
done
clear
B)
\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]and\[{{[Cr{{(CN)}_{6}}]}^{3-}}\]
done
clear
C)
\[{{[Fe{{(CN)}_{6}}]}^{3-}}\]and\[{{[Fe{{(CN)}_{6}}]}^{4-}}\]
done
clear
D)
\[Ni{{(CO)}_{4}}\]and\[{{[Fe{{(CN)}_{6}}]}^{4-}}\]
done
clear
View Answer play_arrow
question_answer 95) Which ammo acid has no asymmetric carbon atom?
A)
Histidine
done
clear
B)
Glycine
done
clear
C)
\[\alpha -\]alanine
done
clear
D)
Threonin
done
clear
View Answer play_arrow
question_answer 96) An organic compound with the formula\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\]forms a yellow crystalline solid with phenylhydrazine and gives a mixture of sorbitol and mannitol when reduced with sodium. Which among the following could be the compound?
A)
Fructose
done
clear
B)
Glucose
done
clear
C)
Mannose
done
clear
D)
Sucrose
done
clear
View Answer play_arrow
question_answer 97) Identify the incorrect statement.
A)
The oxidation of iodide ions by ozone can be used as a method to estimate ozone
done
clear
B)
Bromine does not liberate nitrogen gas from ammonia like chlorine
done
clear
C)
With hot concentrated alkali, chlorine produces the chloride and chlorate
done
clear
D)
Only fluorine attacks\[Si{{O}_{2}}\]to give\[Si{{F}_{3}}\]
done
clear
View Answer play_arrow
question_answer 98) The maximum number of electrons in all those orbitals for which principal quantum number is 3 and azimuthal quantum number 2, is
A)
2
done
clear
B)
8
done
clear
C)
10
done
clear
D)
18
done
clear
View Answer play_arrow
question_answer 99) The weight of hypo\[(N{{a}_{2}}{{S}_{2}}{{O}_{3}}.5{{H}_{2}}O)\]required to make\[100\text{ }c{{m}^{3}}\]of 0.2 N solution for use in the reaction \[2{{S}_{2}}O_{3}^{2-}+{{I}_{2}}\xrightarrow{{}}{{S}_{4}}O_{6}^{2-}+2{{I}^{-}}\]will be
A)
1.49 g
done
clear
B)
2.98 g
done
clear
C)
4.96 g
done
clear
D)
8.94 g
done
clear
View Answer play_arrow
question_answer 100) Which of the following will not show aromatic behaviour?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 101)
Given below are four methods (A-D) and their modes of action (1-4) in achieving contraception. Select their correct matching from the four options that follow. Method Mode of Action A. The pill 1. Prevents sperms Reaching cervix B. Condom 2. Prevents C. Vasectomy 3. Prevents ovulation D. Copper-T 4. Semen contains no sperms
A)
A-3 B-1 C-4 D-2
done
clear
B)
A-4 B-1 C-2 D-3
done
clear
C)
A-3 B-4 C-1 D-2
done
clear
D)
A-2 B-3 C-1 D-4
done
clear
View Answer play_arrow
question_answer 102) Polysome is formed by
A)
several ribosomes attached to a single mRNA
done
clear
B)
many ribosomes attached to a strand of endoplasmic reticulum
done
clear
C)
a ribosome with several subunits
done
clear
D)
ribosomes attached to each other in a linear arrangement
done
clear
View Answer play_arrow
question_answer 103) What is vital capacity of our lungs?
A)
Inspiiatory reserve volume plus tidal volume
done
clear
B)
Total lung capacity minus expiratory reserve volume
done
clear
C)
Inspiratory reserve volume plus expiratory reserve volume
done
clear
D)
Total lung capacity minus residual volume
done
clear
View Answer play_arrow
question_answer 104) Which one of the following is the true description about an animal concerned?
A)
Earthworm ? The alimentary canal consists of a sequence of pharynx, oesophagus, stomach, gizzard and intestine
done
clear
B)
Frog ? Body divisible into three regions-head, neck and trunk
done
clear
C)
Rat? Left kidney is slightly higher in position than the right one
done
clear
D)
Cockroach ? 10 pairs of spiracles (2 pairs on thorax and 8 pairs on abdomen)
done
clear
View Answer play_arrow
question_answer 105) Haploids are more suitable for mutation studies than the diploids. This is because
A)
haploids are reproducrively more stable than diploids
done
clear
B)
mutagenes penetrate in haploids more effectively than in diploids
done
clear
C)
haploids are more abundant in nature than diploids
done
clear
D)
all mutations, whether dominant or recessive are expressed in haploids
done
clear
View Answer play_arrow
question_answer 106) Which one of the following is the correct statement regarding the particular psychotropic drug specified?
A)
Hashish causes alter thought perceptions and hallucinations
done
clear
B)
Opium stimulates nervous system and causes hallucinations
done
clear
C)
Morphine leads to delusions and disturbed emotions
done
clear
D)
Barbiturates cause relaxation and temporary euphoria
done
clear
View Answer play_arrow
question_answer 107) What does the fill form apparatus do at the entrance into ovule?
A)
It helps in the entry of pollen tube into a synergid
done
clear
B)
It prevents entry of more than one pollen tube into the embryo sac
done
clear
C)
It brings about opening of the pollen rube
done
clear
D)
It guides pollen tube from a synergid to egg
done
clear
View Answer play_arrow
question_answer 108) According to Central Pollution Control Board (CPCB), which particulate size in diameter (in micrometres) of the air pollutants is responsible for greatest harm to human health?
A)
2.5 or less
done
clear
B)
1.5 or less
done
clear
C)
1.0 or less
done
clear
D)
5.2 or 2.5
done
clear
View Answer play_arrow
question_answer 109) During the propagation of a nerve impute, the action potential results from die movement of
A)
\[{{K}^{+}}\]ions from extracellular fluid to intracellular fluid
done
clear
B)
\[N{{a}^{+}}\]ions from intracellular fluid to extracellular fluid
done
clear
C)
\[{{K}^{+}}\] ions from intracellular fluid to extracellular fluid
done
clear
D)
\[N{{a}^{+}}\] ions from extracellular fluid to intracellular fluid
done
clear
View Answer play_arrow
question_answer 110) In germinating seeds, fatty acids are degraded exclusively in the
A)
proplastids
done
clear
B)
glyoxysomes
done
clear
C)
peroxisomes
done
clear
D)
mitochondria
done
clear
View Answer play_arrow
question_answer 111) The most active phagocytic white blood cells are
A)
neutrophils and eosinophils
done
clear
B)
lymphocytes and macrophages
done
clear
C)
eosinophils and lymphocytes
done
clear
D)
neutrophils and monocytes
done
clear
View Answer play_arrow
question_answer 112) In humans, blood passes from the post caval to the diastolic right atrium of heart due to
A)
pushing open of the venous valves s
done
clear
B)
suction pull
done
clear
C)
stimulation of the sino auricular node
done
clear
D)
pressure difference between the cava and atrium
done
clear
View Answer play_arrow
question_answer 113) Which one of the following item gives its correct total number?
A)
Floating ribs in humans -4
done
clear
B)
Amino acids found in proteins ? 16
done
clear
C)
Types of diabetes - 3
done
clear
D)
Cervical vertebrae in humans - 8
done
clear
View Answer play_arrow
question_answer 114) Vacuole in a plant cell
A)
is membrane-bound and contains storey proteins and lipids
done
clear
B)
is membrane-bound and contains water and excretory substances
done
clear
C)
lacks membrane and contains air
done
clear
D)
lacks membrane and contains water and excretory substances
done
clear
View Answer play_arrow
question_answer 115) Which one of the following is resistant to enzyme action?
A)
Cork
done
clear
B)
Wood fibre
done
clear
C)
Pollen exine
done
clear
D)
Leaf cuticle
done
clear
View Answer play_arrow
question_answer 116) Which one of the following is not a characteristic of phylum-Annelid a?
A)
Closed circulatory system
done
clear
B)
Segmentation
done
clear
C)
Pseudocoelom
done
clear
D)
Ventral nerve cord
done
clear
View Answer play_arrow
question_answer 117) What is antisense technology?
A)
A cell displaying a foreign antigen used for synthesis of antigens
done
clear
B)
Production of somaclonal variants in tissue cultures
done
clear
C)
When a piece of RNA that is complementary in sequence is used to stop expression of a specific gene
done
clear
D)
RNA polymerase producing DNA
done
clear
View Answer play_arrow
question_answer 118) Importance of day length in flowering of plants was first shown in
A)
Lemna
done
clear
B)
tobacco
done
clear
C)
cotton
done
clear
D)
Petunia
done
clear
View Answer play_arrow
question_answer 119) The two subunits of ribosome remain united at a critical ion level of
A)
copper
done
clear
B)
manganese
done
clear
C)
magnesium
done
clear
D)
calcium
done
clear
View Answer play_arrow
question_answer 120) The energy-releasing process in which the substrate is oxidised without an external electron acceptor is called
A)
fermentation
done
clear
B)
photorespiration
done
clear
C)
aerobic respiration
done
clear
D)
glycolysis
done
clear
View Answer play_arrow
question_answer 121) Cornea transplant in humans is almost never rejected. This is because
A)
its cells are least penetrable by bacteria
done
clear
B)
it has no blood supply
done
clear
C)
it is composed of enucleated cells
done
clear
D)
it is a non-living layer
done
clear
View Answer play_arrow
question_answer 122) \[\text{Cry-I}\] endotoxins obtained from Bacillus thuringiensis are effective against
A)
mosquitoes
done
clear
B)
flies
done
clear
C)
nematodes
done
clear
D)
boll worms
done
clear
View Answer play_arrow
question_answer 123) In which one of the following, the male and female gamerophytes don't have free-living independent existence?
A)
Pteris
done
clear
B)
Funaria
done
clear
C)
Polytrichum
done
clear
D)
Cedrus
done
clear
View Answer play_arrow
question_answer 124) In human adult females, oxytocin
A)
is secreted by anterior pituitary
done
clear
B)
stimulates growth of mammary glands
done
clear
C)
stimulates pituitary to secrete vasopressin
done
clear
D)
causes strong uterine contractions during parturition
done
clear
View Answer play_arrow
question_answer 125) Main objective of production/use of herbicide resistant GM crops is to
A)
eliminate weeds from the field without the use of manual labour
done
clear
B)
eliminate weeds from the field without the use of herbicides
done
clear
C)
encourage eco-friendly herbicides
done
clear
D)
reduce herbicide accumulation in food particles for health safety
done
clear
View Answer play_arrow
question_answer 126) A competitive inhibitor of succinic dehydrogenase is
A)
malonate
done
clear
B)
oxaloacetate
done
clear
C)
\[\text{ }\!\!\alpha\!\!\text{ -}\]ketoglutarate
done
clear
D)
malate
done
clear
View Answer play_arrow
question_answer 127) Which one of the following pair of nitrogenous bases of nucleic acids, is wrongly matched with the category mentioned against it?
A)
Thymine, Uracil - Pyrimidines
done
clear
B)
Uracil, Cytosine - Pyrimidines
done
clear
C)
Guanine, Adenine ? Purines
done
clear
D)
Adenine, Thymine- Purines
done
clear
View Answer play_arrow
question_answer 128) Human insulin is being commercially produced from a transgenic species of
A)
Escherichia
done
clear
B)
Mycobacterium
done
clear
C)
Rhizobium
done
clear
D)
Saccharomyces
done
clear
View Answer play_arrow
question_answer 129) Bacterial leaf blight of rice is caused by a species of
A)
Xanthomonas
done
clear
B)
Pseudomonas
done
clear
C)
Altemaria
done
clear
D)
Erwima
done
clear
View Answer play_arrow
question_answer 130) In humans, at the end of the first meiotic division, the male germ cells differentiate into the
A)
primary spermatocytes
done
clear
B)
secondary spermatocytes
done
clear
C)
spermatids
done
clear
D)
spermatogonia
done
clear
View Answer play_arrow
question_answer 131) Electrons from excited chlorophyll molecule of photosystem-\[\text{II}\] are accepted first by
A)
cytochrome-\[b\]
done
clear
B)
cytochrome-\[f\]
done
clear
C)
quinone
done
clear
D)
ferredoxin
done
clear
View Answer play_arrow
question_answer 132) To which type of barriers under innate immunity, do the saliva in the mouth and the tears from the eyes, belong?
A)
Cytokine barriers
done
clear
B)
Cellular barriers
done
clear
C)
Physiological barriers
done
clear
D)
Physical barriers
done
clear
View Answer play_arrow
question_answer 133) Cellulose is the major component of cell walls of
A)
Pythium
done
clear
B)
Xanthomonas
done
clear
C)
Pseudomonas
done
clear
D)
Saccharomyces
done
clear
View Answer play_arrow
question_answer 134) The slow rate of decomposition of fallen logs in nature is due to their
A)
low moisture content
done
clear
B)
poor nitrogen content
done
clear
C)
anaerobic environment around them
done
clear
D)
low cellulose content
done
clear
View Answer play_arrow
question_answer 135) In leaves of \[{{\text{C}}_{\text{4}}}\text{-}\]plants malic acid synthesis during \[\text{C}{{\text{O}}_{\text{2}}}\text{-}\]fixation occurs in
A)
epidermal cells
done
clear
B)
mesophyll cells
done
clear
C)
bundle sheath
done
clear
D)
guard cells
done
clear
View Answer play_arrow
question_answer 136) Earthworms have no skeleton but during burrowing, the anterior end becomes turgid and acts as a hydraulic skeleton. It is due to
A)
coelomic fluid
done
clear
B)
blood
done
clear
C)
gut peristalsis
done
clear
D)
setae
done
clear
View Answer play_arrow
question_answer 137) Which one of the following pairs of organs includes only the endocrine glands?
A)
Parathyroid and adrenal
done
clear
B)
Pancreas and parathyroid
done
clear
C)
Thymus and testes
done
clear
D)
Adrenal and ovary
done
clear
View Answer play_arrow
question_answer 138) Replum is present in the ovary of flower of
A)
lemon
done
clear
B)
mustard
done
clear
C)
sunflower
done
clear
D)
pea
done
clear
View Answer play_arrow
question_answer 139) Darwin's finches are an excellent example of
A)
adaptive radiation
done
clear
B)
seasonal migration
done
clear
C)
brood parasitism
done
clear
D)
connecting links
done
clear
View Answer play_arrow
question_answer 140) Endosperm is consumed by developing embryo n the seed of
A)
coconut
done
clear
B)
castor
done
clear
C)
pea
done
clear
D)
maize
done
clear
View Answer play_arrow
question_answer 141) Dry indehiscent single-seeded fruit formed from bicarpellary syncarpous inferior ovary is
A)
caryopsis
done
clear
B)
cypsela
done
clear
C)
berry
done
clear
D)
cremocarp
done
clear
View Answer play_arrow
question_answer 142) Nitrogen-fixation in root nodules of Alnus is brought about by
A)
Bradyrhizobium
done
clear
B)
Clostridium
done
clear
C)
Frankia
done
clear
D)
Azorhizobium
done
clear
View Answer play_arrow
question_answer 143) Which one of the following is heterosporous?
A)
Dryopetris
done
clear
B)
Salvinia
done
clear
C)
Adiantum
done
clear
D)
Equisetum
done
clear
View Answer play_arrow
question_answer 144) The \[{{\text{C}}_{\text{4}}}\text{-}\]plants are photosynthetically more efficient than \[{{\text{C}}_{3}}\text{-}\]plants because
A)
the \[C{{O}_{2}}\]compensation point is more
done
clear
B)
\[C{{O}_{2}}\]generated during photorespiralion is trapped and recycled through PEP carboxylase
done
clear
C)
the \[C{{O}_{2}}\]efflux is not prevented
done
clear
D)
they have more chloroplasts
done
clear
View Answer play_arrow
question_answer 145) Which one of the following is linked to the discovery of Bordeaux mixture as a popular fungicide?
A)
Bacterial leaf blight of rice
done
clear
B)
Downy mildew of grapes
done
clear
C)
Loose smut of wheat
done
clear
D)
Black rust of wheat
done
clear
View Answer play_arrow
question_answer 146) Consider the following four measures (A-D) that could be taken to successfully grow dud pea in an area where bacterial blight disease is common. A Spary with Bordeaux mixture. B Control of the insect vector of the disease pathogen. C Use of only disease-free seeds. D Use of varieties resistant to the disease. Which two of the above measures can control the disease?
A)
B and C
done
clear
B)
A and B
done
clear
C)
C and D
done
clear
D)
A and D
done
clear
View Answer play_arrow
question_answer 147) The haemoglobin of a human foetus
A)
has a lower affinity for oxygen than that of the adult
done
clear
B)
its affinity for oxygen is the same as that of an adult
done
clear
C)
has only two protein subunits instead of four
done
clear
D)
has a higher affinity for oxygen than that of an adult
done
clear
View Answer play_arrow
question_answer 148) The rupture and fracdonation do not usually occur in the water column in vessel/tracheids during the ascent of sap becasue of
A)
lignified thick walls
done
clear
B)
cohesion and adhesion
done
clear
C)
weak gravitational pull
done
clear
D)
transpiration pull
done
clear
View Answer play_arrow
question_answer 149) Vascular tissues in flowering plants develop from
A)
phellogen
done
clear
B)
plerome
done
clear
C)
periblem
done
clear
D)
dermatogens
done
clear
View Answer play_arrow
question_answer 150) Which type of white blood cells are concerned with the release of histamine and the natural anticoagulant heparin?
A)
Neutrophils
done
clear
B)
Basophils
done
clear
C)
Eosinophus
done
clear
D)
Monocytes
done
clear
View Answer play_arrow
question_answer 151) The fleshy receptacle of syconous of fig encloses a number of
A)
achenes
done
clear
B)
samaras
done
clear
C)
berries
done
clear
D)
mericarps
done
clear
View Answer play_arrow
question_answer 152) Which extra embryonic membrane in humans prevents desiccation of the embryo inside the uterus?
A)
Chorion
done
clear
B)
Allanlois
done
clear
C)
Yolk sac
done
clear
D)
Amnion
done
clear
View Answer play_arrow
question_answer 153) Modern detergents contain enzyme preparations of
A)
acidophiles
done
clear
B)
alkaliphiles
done
clear
C)
the rmoacidophiles
done
clear
D)
the rmophiles
done
clear
View Answer play_arrow
question_answer 154) The linking of antibiotic resistance gene with the plasmid vector became possible with
A)
DNA ligase
done
clear
B)
endonucleases
done
clear
C)
DNA polymerase
done
clear
D)
exonucleases
done
clear
View Answer play_arrow
question_answer 155) The fruit is chambered, developed from inferior ovary and has seeds with succulent testa in
A)
pomegranate
done
clear
B)
orange
done
clear
C)
guava
done
clear
D)
cucumber
done
clear
View Answer play_arrow
question_answer 156) The centromere or primary constriction of the chromosome contains rings of proteins that are intimately associated with a spindle fibre. These rings are called
A)
centrioles
done
clear
B)
secondary constriction
done
clear
C)
asters
done
clear
D)
kinetochores
done
clear
View Answer play_arrow
question_answer 157) Which of the following is correct order of the evolutionary history of man?
A)
Peking man. Homo sapiens. Neanderthal man, Cro-Magnon man
done
clear
B)
Peking man, Neanderthal man, Homo sapiens, Cro-Magnon man
done
clear
C)
Peking man, Hedelberg man, Neanderthal man, Cro-Magnon man
done
clear
D)
Peking man. Neanderthal man, Homo sapiens, Hedelberg man
done
clear
View Answer play_arrow
question_answer 158) First life on earth was
A)
cyanobacteria
done
clear
B)
chemoheterotrophs
done
clear
C)
autotrophs
done
clear
D)
photoautotrophs
done
clear
View Answer play_arrow
question_answer 159) In negative operon
A)
co-repressor binds with repressor
done
clear
B)
co-repressor does not bind with represser
done
clear
C)
co-repressor binds with inducer
done
clear
D)
c-AMP hs negative effect on lac operon
done
clear
View Answer play_arrow
question_answer 160) Similarities in organisms with different genotypes indicates
A)
microevolution
done
clear
B)
macroevolution
done
clear
C)
convergent evolution
done
clear
D)
divergent evolution
done
clear
View Answer play_arrow
question_answer 161) Sickle cell anaemia is due to
A)
change of amino acid in \[\text{ }\!\!\alpha\!\!\text{ -}\]chain of haemoglobin
done
clear
B)
change of amino add in \[\text{ }\!\!\beta\!\!\text{ -}\]chain of haemoglobin
done
clear
C)
change of amino acid in both \[\text{ }\!\!\alpha\!\!\text{ -}\] and \[\text{ }\!\!\beta\!\!\text{ -}\] chains of haemoglobin
done
clear
D)
change of amino acid in either \[\text{ }\!\!\alpha\!\!\text{ -}\] or \[\text{ }\!\!\beta\!\!\text{ -}\] chain of haemoglobin
done
clear
View Answer play_arrow
question_answer 162) Reason of diversity in living beings is
A)
mutation
done
clear
B)
long term evolutionary change
done
clear
C)
gradual change
done
clear
D)
short term evolutionary change
done
clear
View Answer play_arrow
question_answer 163) Which of the following metal pollution causes sterility in human beings?
A)
Mercury
done
clear
B)
Arsenic
done
clear
C)
Manganese
done
clear
D)
Chromium
done
clear
View Answer play_arrow
question_answer 164) Which of the following is closest relative of man?
A)
Chimpanzee
done
clear
B)
Gorilla
done
clear
C)
Orangutan
done
clear
D)
Gibbon
done
clear
View Answer play_arrow
question_answer 165) In his experiment, Mendel obtained wrinkled pea. The wrinkling was due to deposition of sugar instead of starch. This happened due to the enzyme
A)
amylase
done
clear
B)
invertase
done
clear
C)
diastase
done
clear
D)
absence of starch-branching enzyme
done
clear
View Answer play_arrow
question_answer 166) Which of these do not follow independent assortment?
A)
Genes on non-homologous chromosomes and absence of linkage
done
clear
B)
Genes on homologous chromosomes
done
clear
C)
Linkage genes on same chromosomes
done
clear
D)
Unlinked genes on same chromosome
done
clear
View Answer play_arrow
question_answer 167) A mutant strain of T4-bacteriophage \[\text{R-II,}\] fails to lyse the E.coli but when two strains \[\text{R-I}{{\text{I}}^{\text{X}}}\] and \[\text{R-I}{{\text{I}}^{y}}\] are mixed then they lyse the E.coli. What may be the possible reason?
A)
Bacteriophage transforms in wild
done
clear
B)
It is not mutated
done
clear
C)
Both strains have similar cistrons
done
clear
D)
Both strains have different cistrons
done
clear
View Answer play_arrow
question_answer 168) At the time of organogenesis, genes regulate the process at different levels and at different time due to
A)
promoter
done
clear
B)
regulator
done
clear
C)
intron
done
clear
D)
exon
done
clear
View Answer play_arrow
question_answer 169) Heavy rainfall during summer produces
A)
desert
done
clear
B)
grassland
done
clear
C)
forest
done
clear
D)
wetland
done
clear
View Answer play_arrow
question_answer 170) Which of the following are less general in characters as compared to genus?
A)
Species
done
clear
B)
Division
done
clear
C)
Class
done
clear
D)
Family
done
clear
View Answer play_arrow
question_answer 171) Element necessary for the middle lamella is
A)
Calcium
done
clear
B)
Zinc
done
clear
C)
Potassium
done
clear
D)
Copper
done
clear
View Answer play_arrow
question_answer 172) Extranuclear chromosomes occcur in
A)
peroxisome and ribosome
done
clear
B)
chloroplast and mitochondria
done
clear
C)
mitochondria and ribosome
done
clear
D)
chloroplast and lysosome
done
clear
View Answer play_arrow
question_answer 173) Spoilage of oil can be detected by which fatty acid?
A)
Oleic acid
done
clear
B)
Linolenic acid
done
clear
C)
Linoleic acid
done
clear
D)
Erusic acid
done
clear
View Answer play_arrow
question_answer 174) What is true for archaebacteria?
A)
All are halophilic
done
clear
B)
All are photosynthetic
done
clear
C)
All are fossils
done
clear
D)
These are oldest living beings
done
clear
View Answer play_arrow
question_answer 175) Which one of the following is correct match?
A)
Reserpine - Tranquiliser
done
clear
B)
Cocaine - Opiatic narcotic
done
clear
C)
Morphine - Hallucinogenic
done
clear
D)
Bhang - Analgesic
done
clear
View Answer play_arrow
question_answer 176) Which fish selectively feeds on the larva at mosquito?
A)
Gambusia
done
clear
B)
Rohu
done
clear
C)
Clarias
done
clear
D)
Exocoetus
done
clear
View Answer play_arrow
question_answer 177) Forthcoming generations are less adapting than their parental generation due to
A)
natural selection
done
clear
B)
mutation
done
clear
C)
genetic drift
done
clear
D)
adaptation
done
clear
View Answer play_arrow
question_answer 178) Due to discovery of which of the following id 1980s, the evolution was termed as RNA world?
A)
mRNA, tRNA, rRNA synthesise protein
done
clear
B)
In some viruses, RNA is genetic material
done
clear
C)
Some RNAs have enzymatic property
done
clear
D)
RNA is not found in all cells
done
clear
View Answer play_arrow
question_answer 179) A and B genes are linked. What shall be the genotype of progeny in a cross between AB/ab and ab/ab?
A)
AAbb and aabb
done
clear
B)
AaBb and aabb
done
clear
C)
AABB and aabb
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 180) When dominant and recessive alleles express together, it is called
A)
codominance
done
clear
B)
dominance
done
clear
C)
amphidominance
done
clear
D)
pseudodominance
done
clear
View Answer play_arrow
question_answer 181) Maximum number of bases in plasmid discovered so far is
A)
50 kilobase
done
clear
B)
500 kilobase
done
clear
C)
5,000 kilobase
done
clear
D)
5 kilobase
done
clear
View Answer play_arrow
question_answer 182) Half life period of \[{{C}^{14}}\]is
A)
500 years
done
clear
B)
5,000 years
done
clear
C)
50 years
done
clear
D)
\[5\times {{10}^{14}}\]years
done
clear
View Answer play_arrow
question_answer 183) The end product of Ornithine cycle is
A)
urea
done
clear
B)
uric acid
done
clear
C)
ammonia
done
clear
D)
carbon dioxide
done
clear
View Answer play_arrow
question_answer 184) Which one is correctly matched?
A)
Vitamin-E - Tocopherol
done
clear
B)
Vitamin-D - Riboflavin
done
clear
C)
Vitamin-B - Calciferol
done
clear
D)
Vitamin-A - Thiamine
done
clear
View Answer play_arrow
question_answer 185) In Hydra, waste material of food digestion and nitrogenous waste material are removed from
A)
mouth and mouth
done
clear
B)
body wall and body wall
done
clear
C)
mouth and body wall
done
clear
D)
mouth and tentacles
done
clear
View Answer play_arrow
question_answer 186) Which of the following chamber of heart has the thickest muscular wall?
A)
Left auricle
done
clear
B)
Left ventricle
done
clear
C)
Right ventricle
done
clear
D)
Right auricle
done
clear
View Answer play_arrow
question_answer 187) Which gland plays key role in metamorphosis of frog?
A)
Adrenal
done
clear
B)
Thyroid
done
clear
C)
Thymus
done
clear
D)
Pancreas Thyroid gland secretes thyroxine hormone. It initiates, regulates and plays an important role in metamorphosis of frog.
done
clear
View Answer play_arrow
question_answer 188) Restriction endonucleases are used as
A)
molecular bluid up at nucleotides
done
clear
B)
molecular degradation to DNA breakup
done
clear
C)
molecular knives for cutting DNA at specific sites
done
clear
D)
molecular cement to combine DNA sites
done
clear
View Answer play_arrow
question_answer 189) A plant cell has potential to develop into a full plant. This is called
A)
totipolency
done
clear
B)
gene cloning
done
clear
C)
tissue culture
done
clear
D)
regeneration
done
clear
View Answer play_arrow
question_answer 190) Linnaeus system of classification is
A)
natural
done
clear
B)
artificial
done
clear
C)
phylogenetic
done
clear
D)
progressive
done
clear
View Answer play_arrow
question_answer 191) Number of Ban- bodies in XXXX female
A)
one
done
clear
B)
two
done
clear
C)
three
done
clear
D)
four
done
clear
View Answer play_arrow
question_answer 192) Who coined the term zymase?
A)
Pasteur
done
clear
B)
Buchner
done
clear
C)
Kuhne
done
clear
D)
Sumner
done
clear
View Answer play_arrow
question_answer 193) Which is the example of conditioned reflex?
A)
Eye closed when anything enter into it
done
clear
B)
Hand took up when piercing with needle
done
clear
C)
Your kneeing took up a stone then dog runs away
done
clear
D)
Digestion food goes forward in alimentary canal
done
clear
View Answer play_arrow
question_answer 194) The spice saffron is obtained from which one of the following?
A)
Corolla
done
clear
B)
Stamen
done
clear
C)
Stigma
done
clear
D)
Tender tip of leaf
done
clear
View Answer play_arrow
question_answer 195) The coconut water is a part of
A)
nucellus
done
clear
B)
pericarp
done
clear
C)
embryo
done
clear
D)
endosperm
done
clear
View Answer play_arrow
question_answer 196) Turmeric has recently become widely popular asa
A)
condiment
done
clear
B)
source of insecticides
done
clear
C)
source of antibiotics
done
clear
D)
source of pigments
done
clear
View Answer play_arrow
question_answer 197) A community of organisms interacting with abiotic environmental factors constitutes
A)
a niche
done
clear
B)
a biogeochemical cycle
done
clear
C)
an ecosystem
done
clear
D)
a food chain
done
clear
View Answer play_arrow
question_answer 198) The food chain in which microbes breakdown energy rich compounds synthesised by producers is called
A)
ecosystem
done
clear
B)
parasitic food chain
done
clear
C)
detritus level chain
done
clear
D)
predator food chain
done
clear
View Answer play_arrow
question_answer 199) The function of renin is
A)
vasodilation
done
clear
B)
reduce blood pressure
done
clear
C)
degradation of angiotensinogen
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 200) Heart beat increases at the time of interview because
A)
hypersecretion of renin
done
clear
B)
hyposecretion of renin
done
clear
C)
secretion of adrenaline
done
clear
D)
direlic hormone
done
clear
View Answer play_arrow
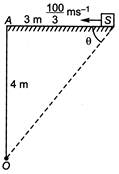 frequency v'. The distance of 0 from A at that time is 4m. If the original frequency is 640 Hz, then the value of v' is (Given that velocity of sound \[=340\text{ }m{{s}^{-1}}\] )
frequency v'. The distance of 0 from A at that time is 4m. If the original frequency is 640 Hz, then the value of v' is (Given that velocity of sound \[=340\text{ }m{{s}^{-1}}\] )