A) \[o.64\times {{10}^{-10}}m\]
B) \[o.56\times {{10}^{-10}}m\]
C) \[o.51\times {{10}^{-10}}m\]
D) \[o.48\times {{10}^{-10}}m\]
Correct Answer: A
Solution :
Key Idea: The cartesian co-ordinate of the centre of mass is \[\frac{\underset{i}{\mathop{\Sigma }}\,{{m}_{i}}{{x}_{i}}}{\Sigma {{m}_{i}}}\] Let the distance of the centre of mass from the carbon atom be r. The mass of carbon, \[{{m}_{1}}=12\text{ }amu\] The mass of oxygen, \[{{m}_{2}}=16amu\]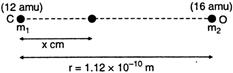 From definition of centre of mass \[{{x}_{cm}}=\frac{{{m}_{1}}{{x}_{1}}+{{m}_{2}}{{x}_{2}}}{{{m}_{1}}+{{m}_{2}}}\] \[=\frac{(12amu)\times 0+(16amu)\times r}{12amu+16amu}\] \[=\frac{16}{28}r=\frac{16}{28}\times 1.12\times {{10}^{-10}}m\] \[=0.64\times {{10}^{-10}}m\]
From definition of centre of mass \[{{x}_{cm}}=\frac{{{m}_{1}}{{x}_{1}}+{{m}_{2}}{{x}_{2}}}{{{m}_{1}}+{{m}_{2}}}\] \[=\frac{(12amu)\times 0+(16amu)\times r}{12amu+16amu}\] \[=\frac{16}{28}r=\frac{16}{28}\times 1.12\times {{10}^{-10}}m\] \[=0.64\times {{10}^{-10}}m\]
You need to login to perform this action.
You will be redirected in
3 sec
