A) 1
B) 2
C) 3
D) 4
Correct Answer: D
Solution :
In ice, each \[H{{e}^{+}}\] molecule is surrounded by four other water molecules, bonded through. H-bonds.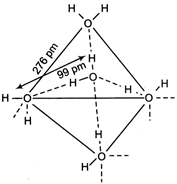 Each \[x\,c{{m}^{-1}}\] molecule linked to four \[4x\,c{{m}^{-1}}\] molecules tetrahedrally.
Each \[x\,c{{m}^{-1}}\] molecule linked to four \[4x\,c{{m}^{-1}}\] molecules tetrahedrally.
You need to login to perform this action.
You will be redirected in
3 sec
