A) \[{{10}^{-4}}\,M\,C{{a}^{2+}}+{{10}^{-4}}M{{F}^{-}}\]
B) \[{{10}^{-2}}\,M\,C{{a}^{2+}}+{{10}^{-3}}M{{F}^{-}}\]
C) \[2{{H}^{+}}+2{{e}^{-}}+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}{{H}_{2}}O(l);{{E}^{o}}=+1.23V\]
D) \[F{{e}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Fe(s);\,\,{{E}^{o}}=-0.44V\]
Correct Answer: D
Solution :
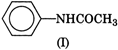
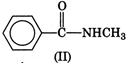
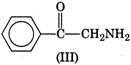
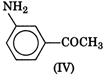
Electron releasing groups increase the basic strength of amines whereas electron withdrawing groups decrease it. Therefore, III > II > IV > I, since inYou need to login to perform this action.
You will be redirected in
3 sec
