| \[\begin{align} & Benzyl\text{ }alcohol \\ & I \\ \end{align}\] |
| \[\begin{align} & Benzoic\text{ }acid \\ & II \\ \end{align}\] |
| \[\begin{align} & o\text{ }-\text{ }cresol \\ & III \\ \end{align}\] |
| \[\begin{align} & Formic\text{ }acid \\ & IV \\ \end{align}\] |
A) \[({{E}^{o}})\]
B) \[OC{{l}^{-}}/C{{l}^{-}}\]
C) \[C{{l}^{-}}/\frac{1}{2}C{{l}_{2}}\]
D) \[1.36\text{ }V\]
Correct Answer: C
Solution :
\[HCOOH\] is stronger acid than \[PhCOOH\]. Aromatic acids are stronger acids than alcohols.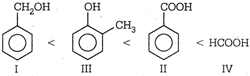
You need to login to perform this action.
You will be redirected in
3 sec
