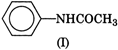
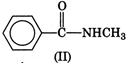
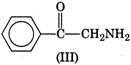
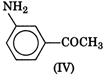
question_answer 1) In a non-resonant circuit, what will be the nature of the circuit for frequencies higher than the resonant frequencies?
A)
Resistive
done
clear
B)
Capacitive
done
clear
C)
Inductive
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 2) A particle of mass m is moving in a circular path of constant radius r such that centripetal acceleration a c varying with time is \[{{10}^{-6}}{{m}^{2}}\], where k is a constant. What is the power delivered to the particle by the force acting on it?
A)
\[{{10}^{29}}\]
done
clear
B)
\[250\times {{10}^{-3}}m/s\]
done
clear
C)
\[25\times {{10}^{-3}}m/s\]
done
clear
D)
\[2.50\times {{10}^{-3}}m/s\]
done
clear
View Answer play_arrow
question_answer 3) Rain is falling vertically downwards with a velocity of 4 km/h. A man walks in the rain with a velocity of 3 km/h. The raindrops will falls on the man with a velocity of
A)
\[1.25\times {{10}^{3}}m/s\]
done
clear
B)
\[\frac{hv}{4}\]
done
clear
C)
\[\frac{hv}{3}\]
done
clear
D)
\[\frac{hv}{2}\]
done
clear
View Answer play_arrow
question_answer 4) Change in frequency due to Dopplers effect is produced when,
A)
the source and the observer are moving: in same direction
done
clear
B)
the source and the observer are both at rest
done
clear
C)
there is a relative motion between the source and the observer
done
clear
D)
there is a resultant motion between the source and observer
done
clear
View Answer play_arrow
question_answer 5) There is a current of 40 A in a wire of \[\frac{2hv}{3}\] area of cross-section. If the number of free electrons per cubic metre is \[30\mu A\], then the drift velocity is
A)
\[90\mu A\]
done
clear
B)
\[4mA\]
done
clear
C)
\[2mA\]
done
clear
D)
\[3.6mA\]
done
clear
View Answer play_arrow
question_answer 6) The velocity of the most energetic electrons emitted from a metallic surface is doubled when the frequency v of the incident radiation is doubled. The work function of this metal is
A)
\[1.8mA\]
done
clear
B)
\[62V,\,\,2\Omega \]
done
clear
C)
\[63V,\,\,1\Omega \]
done
clear
D)
\[61V,\,\,1\Omega \]
done
clear
View Answer play_arrow
question_answer 7) In a transistor circuit, the base current changes from \[64V,\,\,2\Omega \] to \[15min\]. If the current gain of the transistor is 30, the change in the collector current is
A)
\[20\text{ }min\]
done
clear
B)
\[7.5min\]
done
clear
C)
\[25min\]
done
clear
D)
\[\frac{\omega M}{M+m}\]
done
clear
View Answer play_arrow
question_answer 8) The potential difference across the terminals of a battery is 50V when 11 A current is drawn and 60V when 1 A current is drawn. The emf and the internal resistance of the battery, are
A)
\[\frac{\omega M}{M+m}\]
done
clear
B)
\[{{a}_{c}}={{k}^{2}}{{r}^{2}}{{t}^{2}},\]
done
clear
C)
\[2mk{{r}^{2}}t\]
done
clear
D)
\[mk{{r}^{2}}{{t}^{2}}\]
done
clear
View Answer play_arrow
question_answer 9) Two similar heater coils separately take 10 min to boil a certain amount of water. If both coils are connected in series, then time taken to boil the same amount of water will be
A)
\[m{{k}^{2}}{{r}^{2}}t\]
done
clear
B)
\[m{{k}^{2}}r{{t}^{2}}\]
done
clear
C)
\[1\text{ }km/h\]
done
clear
D)
\[\text{3 }km/h\]
done
clear
View Answer play_arrow
question_answer 10) A thin circular ring of mass M and radius R is rotating about its axis with a constant angular velocity co. Two objects each of mass m are connected gently to the ring. The ring now rotates with an angular velocity of
A)
\[\text{4 }km/h\]
done
clear
B)
\[\text{5 }km/h\]
done
clear
C)
\[{{10}^{-6}}{{m}^{2}}\]
done
clear
D)
\[{{10}^{29}}\]
done
clear
View Answer play_arrow
question_answer 11) When a van der Waals gas undergoes free expansion, then its temperature
A)
decreases
done
clear
B)
increases
done
clear
C)
does not change
done
clear
D)
depends upon the nature of the gas
done
clear
View Answer play_arrow
question_answer 12)
In a gravitational force field, a particle is taken from A to B along different paths as shown in figure. Then,
A)
work done along path I will be maximum
done
clear
B)
work done along path III will be maximum
done
clear
C)
work done along path IV will be maximum
done
clear
D)
work done along all the paths will be the same
done
clear
View Answer play_arrow
question_answer 13) When 1 cm thick surface is illuminated with light of wavelength \[250\times {{10}^{-3}}m/s\], the stopping potential is V. When the same surface is illuminated by light of wavelength \[25\times {{10}^{-3}}m/s\], the stopping potential is \[2.50\times {{10}^{-3}}m/s\]. Threshold wavelength for metallic surface is
A)
\[1.25\times {{10}^{3}}m/s\]
done
clear
B)
\[\frac{hv}{4}\]
done
clear
C)
\[\frac{hv}{3}\]
done
clear
D)
\[\frac{hv}{2}\]
done
clear
View Answer play_arrow
question_answer 14) The Reynolds number of a flow is the ratio of
A)
gravity to viscous force
done
clear
B)
gravity force to pressure force
done
clear
C)
inertia force to viscous force
done
clear
D)
viscous force to pressure force
done
clear
View Answer play_arrow
question_answer 15)
The equivalent resistance between the points X and Y in the circuit shown is
A)
\[\frac{2hv}{3}\]
done
clear
B)
\[30\mu A\]
done
clear
C)
\[90\mu A\]
done
clear
D)
\[4mA\]
done
clear
View Answer play_arrow
question_answer 16) A projectile is thrown in the upward direction making an angle of \[2mA\] with the horizontal direction with a velocity of 147 m/s. Then, the time after which its inclination with the horizontal is \[3.6mA\], is
A)
\[1.8mA\]
done
clear
B)
\[62V,\,\,2\Omega \]
done
clear
C)
\[63V,\,\,1\Omega \]
done
clear
D)
\[61V,\,\,1\Omega \]
done
clear
View Answer play_arrow
question_answer 17) The angle of minimum deviation for a glass prism is equal to its refracting angle. The refractive index of the glass is 1.5. Then, the! angle of prism is
A)
\[64V,\,\,2\Omega \]
done
clear
B)
\[15min\]
done
clear
C)
\[20\text{ }min\]
done
clear
D)
\[7.5min\]
done
clear
View Answer play_arrow
question_answer 18) A square of side 3 cm is located at a distance of 25 cm from a concave of square, is at the axis of the mirror and the plane is normal to axis of mirror. The area enclosed by the image of the square is
A)
\[25min\]
done
clear
B)
\[\frac{\omega M}{M+m}\]
done
clear
C)
\[\frac{\omega (M-2M)}{M+2m}\]
done
clear
D)
\[\frac{\omega (M-2M)}{M+2m}\]
done
clear
View Answer play_arrow
question_answer 19) Solar spectrum at the time of total solar eclipse is
A)
line emission spectrum
done
clear
B)
line absorption spectrum
done
clear
C)
continuous emission spectrum
done
clear
D)
band absorption spectrum
done
clear
View Answer play_arrow
question_answer 20) A stone is dropped into a well, if the depth of water below the top be h and the velocity of sound is \[{{a}_{c}}={{k}^{2}}{{r}^{2}}{{t}^{2}},\], then the splash in water is hear after T sec. Then,
A)
\[2mk{{r}^{2}}t\]
done
clear
B)
\[mk{{r}^{2}}{{t}^{2}}\]
done
clear
C)
\[m{{k}^{2}}{{r}^{2}}t\]
done
clear
D)
\[m{{k}^{2}}r{{t}^{2}}\]
done
clear
View Answer play_arrow
question_answer 21) Zener diodes are used for
A)
rectification
done
clear
B)
amplification
done
clear
C)
stabilization
done
clear
D)
production of carries waves
done
clear
View Answer play_arrow
question_answer 22) Forces 5N, 12N and 13N are in equilibrium If \[1\text{ }km/h\]. then the angle between 5N force and 13N force is
A)
\[\text{3 }km/h\]
done
clear
B)
\[\text{4 }km/h\]
done
clear
C)
\[\text{5 }km/h\]
done
clear
D)
\[{{10}^{-6}}{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 23) The angular speed of the earths rotation about its axis is \[{{10}^{29}}\]. Its speed increases x times to make effective acceleration due to gravity equal to zero. At the equator, x is
A)
\[250\times {{10}^{-3}}m/s\]
done
clear
B)
\[25\times {{10}^{-3}}m/s\]
done
clear
C)
\[2.50\times {{10}^{-3}}m/s\]
done
clear
D)
\[1.25\times {{10}^{3}}m/s\]
done
clear
View Answer play_arrow
question_answer 24) Two resistors \[\frac{hv}{4}\]. and \[\frac{hv}{3}\]. are connected in series with 6V battery. The potential difference measured by voltmeter of \[\frac{hv}{2}\] across \[\frac{2hv}{3}\], resistor is
A)
\[30\mu A\]
done
clear
B)
\[90\mu A\]
done
clear
C)
\[4mA\]
done
clear
D)
\[2mA\]
done
clear
View Answer play_arrow
question_answer 25) In a cassette player, materials used for coating magnetic tapes are
A)
cobalt
done
clear
B)
\[3.6mA\]
done
clear
C)
nickel
done
clear
D)
iron
done
clear
View Answer play_arrow
question_answer 26) Mass of 2 kg is moving with a string in horizontal circle with angular velocity 5 cycle/min. Keeping the radius constant, tension in string is doubled. Now, the angular velocity of the mass will be
A)
\[1.8mA\]
done
clear
B)
\[62V,\,\,2\Omega \]
done
clear
C)
\[63V,\,\,1\Omega \]
done
clear
D)
\[61V,\,\,1\Omega \]
done
clear
View Answer play_arrow
question_answer 27) If the number of turns in moving coil galvanometer become half, then the deflection for the same current will become
A)
same
done
clear
B)
half
done
clear
C)
double
done
clear
D)
four times
done
clear
View Answer play_arrow
question_answer 28) 40 cal of heat is required to raise the temperature of 2 mol of an ideal gas at constant pressure from \[64V,\,\,2\Omega \] to \[15min\]. The amount of heat required to raise the temperature of the same. Sample of the gas through the same range of temperature (\[20\text{ }min\]to\[7.5min\] ) at constant volume will be (gas constant = 2 cal/mol)
A)
\[25min\]
done
clear
B)
\[\frac{\omega M}{M+m}\]
done
clear
C)
\[\frac{\omega (M-2M)}{M+2m}\]
done
clear
D)
\[\frac{\omega (M+2M)}{M}\]
done
clear
View Answer play_arrow
question_answer 29) A Light Emitting Diode (LED) has a voltage drop of 2V across it and passes a current of 10mA, when it operates with a 6V battery through a limiting resistor R, the value of R is
A)
\[\frac{\omega (M+2M)}{M}\]
done
clear
B)
\[{{a}_{c}}={{k}^{2}}{{r}^{2}}{{t}^{2}},\]
done
clear
C)
\[2mk{{r}^{2}}t\]
done
clear
D)
\[mk{{r}^{2}}{{t}^{2}}\]
done
clear
View Answer play_arrow
question_answer 30) The dimension of \[m{{k}^{2}}{{r}^{2}}t\](\[m{{k}^{2}}r{{t}^{2}}\]=Stefans constant and b = Weins constant) are
A)
\[1\text{ }km/h\]
done
clear
B)
\[\text{3 }km/h\]
done
clear
C)
\[\text{4 }km/h\]
done
clear
D)
\[\text{5 }km/h\]
done
clear
View Answer play_arrow
question_answer 31) When \[{{10}^{-6}}{{m}^{2}}\] electrons are removed from a neutral metal place plate, the electric charge on it is
A)
\[{{10}^{29}}\]
done
clear
B)
\[250\times {{10}^{-3}}m/s\]
done
clear
C)
\[25\times {{10}^{-3}}m/s\]
done
clear
D)
\[2.50\times {{10}^{-3}}m/s\]
done
clear
View Answer play_arrow
question_answer 32) A coil of cross-sectional area \[1.25\times {{10}^{3}}m/s\] having 30 turns is making 1800 rev/min in a magnetic field of 1 T. The peak value of the induced emf is
A)
\[\frac{hv}{4}\]
done
clear
B)
\[\frac{hv}{3}\]
done
clear
C)
\[\frac{hv}{2}\]
done
clear
D)
\[\frac{2hv}{3}\]
done
clear
View Answer play_arrow
question_answer 33) A body of length 1 m having cross-sectional area \[30\mu A\] has heat flow through it at the rate of 6000 J/s. Then, find the temperature difference if \[90\mu A\].
A)
\[4mA\]
done
clear
B)
\[2mA\]
done
clear
C)
\[3.6mA\]
done
clear
D)
\[1.8mA\]
done
clear
View Answer play_arrow
question_answer 34) A motor cycle racer takes a round with speed of 20m/s in a curvature of radius of R=40 m, then the leaning angle of motor cycle for safe turn is(\[62V,\,\,2\Omega \])
A)
\[63V,\,\,1\Omega \]
done
clear
B)
\[61V,\,\,1\Omega \]
done
clear
C)
\[64V,\,\,2\Omega \]
done
clear
D)
\[15min\]
done
clear
View Answer play_arrow
question_answer 35) Gauss law of gravitation is
A)
\[20\text{ }min\]
done
clear
B)
\[7.5min\]
done
clear
C)
\[25min\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 36) Three masses of 2 kg, 4 kg and 4 kg are placed at the three points (1, 0, 0), (1, 1, 0) and (0, 1, 0) respectively. The position vector of its centre of mass is
A)
\[\frac{\omega M}{M+m}\]
done
clear
B)
\[\frac{\omega (M-2M)}{M+2m}\]
done
clear
C)
\[\frac{\omega (M+2M)}{M}\]
done
clear
D)
\[\frac{\omega M}{M+2m}\]
done
clear
View Answer play_arrow
question_answer 37) For which of the following process is the entropy charge zero?
A)
Isobaric
done
clear
B)
Isothermal
done
clear
C)
Adiabatic
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 38) Two pendulums have time periods T and \[\frac{\omega M}{M+2m}\] They starts SHM at the same time from the mean position. What will be the phase difference between them after the bigger pendulum completed one oscillation?
A)
\[{{a}_{c}}={{k}^{2}}{{r}^{2}}{{t}^{2}},\]
done
clear
B)
\[2mk{{r}^{2}}t\]
done
clear
C)
\[mk{{r}^{2}}{{t}^{2}}\]
done
clear
D)
\[m{{k}^{2}}{{r}^{2}}t\]
done
clear
View Answer play_arrow
question_answer 39) The value of P so that the vectors \[m{{k}^{2}}r{{t}^{2}}\], \[1\text{ }km/h\] and 3i+Pj+5k are coplanar should be
A)
\[\text{3 }km/h\]
done
clear
B)
\[\text{4 }km/h\]
done
clear
C)
\[\text{5 }km/h\]
done
clear
D)
\[{{10}^{-6}}{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 40) Application of a forward bias to a p-n junction
A)
increases the number of donors on the n-side
done
clear
B)
increases the electric field in the depletion zone
done
clear
C)
increases the potential difference across the depletion zone
done
clear
D)
hidden the depletion zone
done
clear
View Answer play_arrow
question_answer 41) A particle moves in xy-plane according to rule \[{{10}^{29}}\] and \[250\times {{10}^{-3}}m/s\]. The particle follows
A)
an elliptical path
done
clear
B)
a circular path
done
clear
C)
a parabolic path
done
clear
D)
a straight line path inclined equally to x and y-axis
done
clear
View Answer play_arrow
question_answer 42) What is the angular velocity of the earth?
A)
\[25\times {{10}^{-3}}m/s\]
done
clear
B)
\[2.50\times {{10}^{-3}}m/s\]
done
clear
C)
\[1.25\times {{10}^{3}}m/s\]
done
clear
D)
\[\frac{hv}{4}\]
done
clear
View Answer play_arrow
question_answer 43) The temperature coefficient of resistance of a wire is \[\frac{hv}{3}\]. Its resistance is \[\frac{hv}{2}\]. At 300 K resistance will be \[\frac{2hv}{3}\], at
A)
\[30\mu A\]
done
clear
B)
\[90\mu A\]
done
clear
C)
\[4mA\]
done
clear
D)
\[2mA\]
done
clear
View Answer play_arrow
question_answer 44) To send 10% of the main current through a moving coil galvanometer of resistance \[3.6mA\], the shunt required is
A)
\[1.8mA\]
done
clear
B)
\[62V,\,\,2\Omega \]
done
clear
C)
\[63V,\,\,1\Omega \]
done
clear
D)
\[61V,\,\,1\Omega \]
done
clear
View Answer play_arrow
question_answer 45) In Millikans oil drop experiment, a charged drop of mass \[64V,\,\,2\Omega \] is stationary between the plates. The distance between the plates is 0.9 cm and potential difference between the plates is 2000 V. The number of electrons on the oil drop is
A)
\[15min\]
done
clear
B)
\[20\text{ }min\]
done
clear
C)
\[7.5min\]
done
clear
D)
\[25min\]
done
clear
View Answer play_arrow
question_answer 46) White light is used to illuminate the two slits in a Youngs double slit experiment. The separation between the slits is b and the screen is at a distance d (> b) from the slits, At a point on the screen directly in front of one of slits certain wavelength are missing. One of these missing wavelength for n = 0 is
A)
\[\frac{\omega M}{M+m}\]
done
clear
B)
\[\frac{\omega (M-2M)}{M+2m}\]
done
clear
C)
\[\frac{\omega (M+2M)}{M}\]
done
clear
D)
\[\frac{\omega M}{M+2m}\]
done
clear
View Answer play_arrow
question_answer 47) If for a gas, \[{{a}_{c}}={{k}^{2}}{{r}^{2}}{{t}^{2}},\], then this gas is made up molecules which are
A)
monoatomic
done
clear
B)
diatomic
done
clear
C)
polyatomic
done
clear
D)
mixture of diatomic and polyatomic molecules
done
clear
View Answer play_arrow
question_answer 48) A simple pendulum with length L and mass m of the bob is vibrating with an amplitude a. Then, the maximum tension in the string is
A)
\[2mk{{r}^{2}}t\]
done
clear
B)
\[mk{{r}^{2}}{{t}^{2}}\]
done
clear
C)
\[m{{k}^{2}}{{r}^{2}}t\]
done
clear
D)
\[m{{k}^{2}}r{{t}^{2}}\]
done
clear
View Answer play_arrow
question_answer 49) If elements with principal quantum numbers n > 4 were not allowed in nature, then the number of possible elements would be
A)
\[1\text{ }km/h\]
done
clear
B)
\[\text{3 }km/h\]
done
clear
C)
\[\text{4 }km/h\]
done
clear
D)
\[\text{5 }km/h\]
done
clear
View Answer play_arrow
question_answer 50) A radioactive elements has half-life period of 600 yr. After 3000 yr, what amount will be remaining?
A)
\[{{10}^{-6}}{{m}^{2}}\]
done
clear
B)
\[{{10}^{29}}\]
done
clear
C)
\[250\times {{10}^{-3}}m/s\]
done
clear
D)
\[25\times {{10}^{-3}}m/s\]
done
clear
View Answer play_arrow
question_answer 51) The Edison storage cell is represented as \[{{O}_{2}}\]\[{{U}_{rms}}{{O}_{2}}=2\times {{U}_{rms}}O\] \[{{U}_{rms}}{{O}_{2}}=\frac{1}{2}\times {{U}_{rms}}O\]\[{{U}_{rms}}{{O}_{2}}=\frac{1}{3}\times {{U}_{rms}}\] What is the maximum amount of electrical energy that can be obtained from one mole of \[{{U}_{rms}}{{O}_{2}}={{U}_{rms}}O\]?
A)
\[Cs-Cl\]
done
clear
B)
\[CsCl\]
done
clear
C)
\[Cl\]
done
clear
D)
\[62.92%\]
done
clear
View Answer play_arrow
question_answer 52) For the reaction, \[60.0%\]\[75.04%\] concentration of \[52.14%\] at the equivalence point in the titration of \[I_{3}^{-}\] of \[3,2\] with \[2,3\] when final volume is 100 mL, is
A)
\[2,1\]
done
clear
B)
\[1,2\]
done
clear
C)
\[x\,c{{m}^{-1}}\]
done
clear
D)
\[H{{e}^{+}}\]
done
clear
View Answer play_arrow
question_answer 53) In a 0.2 molal aqueous solution of a weak acid HX, the degree of ionisation is 0.3. Taking \[B{{e}^{3+}}\] for water as 1.85, the freezing point of the solution will be nearest to
A)
\[H{{e}^{+}}\]
done
clear
B)
\[x\,c{{m}^{-1}}\]
done
clear
C)
\[4x\,c{{m}^{-1}}\]
done
clear
D)
\[\frac{x}{4}\,c{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 54) For an isomerisation reaction \[2x\,c{{m}^{-1}}\], the temperature dependence of equilibrium constant is given by \[O=16,Cu=63,N=14\] The value of \[1\times {{10}^{-10}}g\] at 300 K is therefore,
A)
4R
done
clear
B)
4R
done
clear
C)
400R
done
clear
D)
2000R
done
clear
View Answer play_arrow
question_answer 55) In the Freundlich adsorption equation \[1\times {{10}^{-10}}g\]the value of n is
A)
always greater than one
done
clear
B)
always smaller than one
done
clear
C)
always equal to one
done
clear
D)
greater than one at low temperature and smaller than one at high temperature
done
clear
View Answer play_arrow
question_answer 56) Reaction,\[II<I<\text{ }III<IV\], is used for commercial preparation of bromine from its salts. Suppose we have 50 mL of a 0.06 M solution of \[II<\text{ }III<I<IV\]. What volume of a 0.05 M solution of \[III<IV<I<II\] is needed to react completely with the \[BaC{{l}_{2}}(s)\]?
A)
\[BaC{{l}_{2}}.2{{H}_{2}}O(s)\]
done
clear
B)
\[-20.6\]
done
clear
C)
\[8.8\text{ }kJ\]
done
clear
D)
\[BaC{{l}_{2}}(s)+2{{H}_{2}}O\xrightarrow{{}}BaC{{l}_{2}}.2{{H}_{2}}O(s),\]
done
clear
View Answer play_arrow
question_answer 57) How many Cs atoms can be converted to \[29.4kJ\] ion by 1 J energy if \[-29.4kJ\] for Cs is 376 kJ \[-11.8kJ\]?
A)
\[38.2kJ\]
done
clear
B)
\[{{10}^{o}}C\]
done
clear
C)
\[{{25}^{o}}C\]
done
clear
D)
\[105.3\]
done
clear
View Answer play_arrow
question_answer 58) Among the following the number of compounds that can react with \[{{25}^{o}}C\], to give\[{{V}_{final}}=5{{V}_{initial}}\] is \[{{V}_{initial}}>{{V}_{final}}\]
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 59)
The oxidation state of cobalt in
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 60)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 61) The maximum possible number of hydrogen bonds a water molecule can form in ice is
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 62) Terylene is a condensation polymer of ethylene glycol and
A)
benzoic acid
done
clear
B)
phthalic acid
done
clear
C)
salicylic acid
done
clear
D)
terephthalic acid
done
clear
View Answer play_arrow
question_answer 63) In Rosenmund reaction,\[{{\text{V}}_{\text{final}}}\text{=4}{{\text{V}}_{\text{intial}}}\]\[{{V}_{final}}=6{{V}_{\operatorname{int}ial}}\] here,
A)
promotes catalytic activity of Pd
done
clear
B)
removes the \[Ca{{F}_{2}}({{K}_{sp}}=1.7\times {{10}^{-10}})\] formed in the reaction
done
clear
C)
deactivates palladium
done
clear
D)
activates palladium
done
clear
View Answer play_arrow
question_answer 64)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 65) D-glucose contains
A)
50% each of \[\text{ }\!\!\alpha\!\!\text{ -}\]D-glucose and \[\text{ }\!\!\beta\!\!\text{ -}\]D-glucose
done
clear
B)
60% of \[\text{ }\!\!\alpha\!\!\text{ -}\]D-glucose and 36% of \[\text{ }\!\!\beta\!\!\text{ -}\]D-glucose
done
clear
C)
36% of \[\text{ }\!\!\alpha\!\!\text{ -}\]D-glucose and 64% of \[\text{ }\!\!\beta\!\!\text{ -}\]D-glucose
done
clear
D)
33% of each \[\text{ }\!\!\alpha\!\!\text{ -}\]D-glucose and \[\text{ }\!\!\beta\!\!\text{ -}\]D-glucose
done
clear
View Answer play_arrow
question_answer 66) Which one of the following is the most reactive towards ring nitration?
A)
Benzene
done
clear
B)
Mesitylene
done
clear
C)
Toluene
done
clear
D)
m-xylene
done
clear
View Answer play_arrow
A)
\[{{10}^{-4}}\,M\,C{{a}^{2+}}+{{10}^{-4}}M{{F}^{-}}\]
done
clear
B)
\[{{10}^{-2}}\,M\,C{{a}^{2+}}+{{10}^{-3}}M{{F}^{-}}\]
done
clear
C)
\[2{{H}^{+}}+2{{e}^{-}}+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}{{H}_{2}}O(l);{{E}^{o}}=+1.23V\]
done
clear
D)
\[F{{e}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Fe(s);\,\,{{E}^{o}}=-0.44V\]
done
clear
View Answer play_arrow
question_answer 68) Both HCHO and \[\Delta G\] give similar reactions with all the reagents except
A)
Schiffs reagent
done
clear
B)
Fehling solution
done
clear
C)
ammonia cal \[-322\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
D)
ammonia
done
clear
View Answer play_arrow
question_answer 69) At a temperature T, the \[-161\text{ }kJ\text{ }mo{{l}^{-1}}\] speed of \[-152\,kJ\,\,mo{{l}^{-1}}\] molecules is u. If the temperature is doubled, so that \[-76\,kJ\,mo{{r}^{-1}}\] dissociates into O atoms, then \[-161\text{ }kJ\text{ }mo{{l}^{-1}}\]of oxygen atoms is
A)
\[5mL\]
done
clear
B)
\[A{{s}_{2}}{{S}_{3}}\]
done
clear
C)
\[2Ag+2HCl+(O)\xrightarrow{{}}2AgCl+{{H}_{2}}O\]
done
clear
D)
\[2Ag+2HCl+(O)\xrightarrow{{}}2AgCl+{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 70) The percentage ionic character in \[N{{i}_{2}}{{O}_{2}}(s)+{{H}_{2}}O(l)+2{{e}^{-}}2NiO(s)+2O{{H}^{-}}\] bond present in \[{{E}^{o}}=+0.40V\] molecule will be, if the electronegative values of Cs and \[FeO(s)+{{H}_{2}}O(l)+2{{e}^{-}}Fe(s)+2O{{H}^{-}};\] are 0.8 and 3.0 respectively
A)
\[{{E}^{o}}=-8.87V\]
done
clear
B)
\[N{{i}_{2}}{{O}_{3}}\]
done
clear
C)
\[127\,kJ\]
done
clear
D)
\[245.11kJ\]
done
clear
View Answer play_arrow
question_answer 71) The number of lone pair and bond pair electrons present in central iodine atom of \[90.71kJ\] ion are respectively
A)
\[122.55kJ\]
done
clear
B)
\[H{{g}^{2+}}+2C{{l}^{-}}HgC{{l}_{2}},\]
done
clear
C)
\[k=1.65\times {{10}^{3}}\]
done
clear
D)
\[H{{g}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 72) Wave number of a spectral line for a given transition is \[2.0m\text{ }mol\] for \[H{{g}^{2+}}\] then its value for \[C{{l}^{-}}\] (is electronic of \[825\times {{10}^{14}}M\] ) for the same transition is
A)
\[1.65\times {{10}^{13}}M\]
done
clear
B)
\[2.87\times {{10}^{6}}M\]
done
clear
C)
\[6.72\times {{10}^{6}}M\]
done
clear
D)
\[{{K}_{f}}\]
done
clear
View Answer play_arrow
question_answer 73)
Rearrange the following (I to TV) in the order of increasing masses and choose the correct answer (atomic mass; \[-{{0.360}^{o}}C\]) I. 1 molecule of oxygen II. 1 atom of nitrogen III. \[-{{0.260}^{o}}C\] molecular weight of oxygen IV. \[2.87\times {{10}^{6}}M\] atomic weight of copper
A)
\[672\times {{10}^{6}}M\]
done
clear
B)
IV < III < II < I
done
clear
C)
\[AB\]
done
clear
D)
\[{{\log }_{e}}\,\,K=4.0-\frac{2000}{T}\]
done
clear
View Answer play_arrow
question_answer 74) The enthalpy of dissolution of \[\Delta {{S}^{o}}\] and \[\frac{x}{m}=k{{p}^{1/n}},\] are \[2B{{r}^{-}}(aq)+C{{l}_{2}}(aq)\to 2C{{l}^{-}}(aq)+B{{r}_{2}}(aq)\] and \[NaBr\] per mole respectively. The enthalpy of hydration for, \[C{{l}_{2}}\]is
A)
\[B{{r}^{-}}\]
done
clear
B)
\[50mL\]
done
clear
C)
\[1200mL\]
done
clear
D)
\[30mL\]
done
clear
View Answer play_arrow
question_answer 75) At \[60mL\], the osmotic pressure of urea solution is 500 mm. The solution is diluted and the temperature is raised to \[C{{s}^{+}}\]. The osmotic pressure of dilute solution is \[I{{E}_{1}}\]mm at \[mo{{l}^{-1}}\]. The extent of dilution can be shown as
A)
\[1.60\times {{10}^{23}}\]
done
clear
B)
\[1.60\times {{10}^{15}}\]
done
clear
C)
\[1.60\times {{10}^{18}}\]
done
clear
D)
\[16.0\times {{10}^{26}}\]
done
clear
View Answer play_arrow
question_answer 76) The precipitate of \[PC{{l}_{5}}\] is obtained when equal volumes of the following are mixed
A)
\[POC{{l}_{3}}\]
done
clear
B)
\[{{O}_{2}},C{{O}_{2}},S{{O}_{2}},{{H}_{2}}O,{{H}_{2}}S{{O}_{4}},{{P}_{4}}{{O}_{10}}\]
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 77) The rusting of iron takes place as follows \[RCOCl+{{H}_{2}}\xrightarrow{Pd/BaS{{O}_{4}}}RCHO+HCl\] \[BaS{{O}_{4}}\] Calculate \[HCl\] for the net process.
A)
\[l>II>lll>IV\]
done
clear
B)
\[IV>ll>lll>l\]
done
clear
C)
\[lll>IV>H>l\]
done
clear
D)
\[lll>ll>IV>l\]
done
clear
View Answer play_arrow
question_answer 78) In a coagulating experiment, \[C{{H}_{3}}CHO\] of \[AgN{{o}_{3}}\] is mixed with distilled water and 0.2 M solution of an electrolyte AB, so that the total volume is 20 mL. All solutions containing 5.4 mL of AB coagulate within 2 min. The flocculation value sof AB (in millimol) is
A)
5
done
clear
B)
50
done
clear
C)
54
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 79) The statement that is not correct for the periodic classification of elements, is
A)
the properties of the elements are the periodic function of their atomic number
done
clear
B)
non-metallic elements are lesser in number than metallic elements
done
clear
C)
the first ionisation energies of elements along a period do not vary in a regular manner with increase in atomic number
done
clear
D)
for transition elements the d subshells are filled with electrons monotonically with increase in atomic numbers
done
clear
View Answer play_arrow
question_answer 80) Which one of the following reactions is an example of calcination process?
A)
\[{{u}_{rms}}\]
done
clear
B)
\[{{O}_{2}}\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[{{U}_{rms}}{{O}_{2}}=2\times {{U}_{rms}}O\]
done
clear
View Answer play_arrow
question_answer 81) On dissolving moderate amount of sodium metal in liquid \[{{U}_{rms}}{{O}_{2}}=\frac{1}{2}\times {{U}_{rms}}O\] at low temperature, which one of the following does not occur?
A)
Blue coloured solution is obtained
done
clear
B)
\[{{U}_{rms}}{{O}_{2}}=\frac{1}{3}\times {{U}_{rms}}\] ions are formed in the solution
done
clear
C)
Liquid \[{{U}_{rms}}{{O}_{2}}={{U}_{rms}}O\] solution becomes good conductor of electricity
done
clear
D)
Liquid \[Cs-Cl\] solution remains diamagnetic
done
clear
View Answer play_arrow
question_answer 82) A mixture contains two moles of \[CsCl\] and 1 mole of \[Cl\]. What will be the volume of \[62.92%\] formed on heating this mixture and the data is converted to STP?
A)
\[60.0%\]
done
clear
B)
\[75.04%\]
done
clear
C)
\[52.14%\]
done
clear
D)
\[I_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 83) Aryl halides are less reactive than alkyl halides towards nucleophile due to
A)
resonance
done
clear
B)
stability of carbonium ion
done
clear
C)
high boiling point
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 84) Which of the following statement is wrong?
A)
Polar Stratospheric Clouds (PSCs) are clouds formed over Antarctica
done
clear
B)
Acid rain dissolves heavy metals such as \[3,2\] and \[2,3\] from soil, rocks and sediments
done
clear
C)
\[2,1\] is major contributor to acid rain, \[1,2\] ranks second and HC! third in this respect
done
clear
D)
Fishes grow as well in warm as in cold water
done
clear
View Answer play_arrow
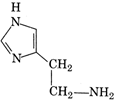
question_answer 85)
The drug is used as
A)
antacid
done
clear
B)
analgesics
done
clear
C)
vasodilator
done
clear
D)
antiseptic
done
clear
View Answer play_arrow
question_answer 86) An organic compound of molecular formula \[x\,c{{m}^{-1}}\] does not react with sodium With excess of \[H{{e}^{+}}\] it gives only one type of alkyl halide. The compound is
A)
ethoxy ethane
done
clear
B)
1-butanol
done
clear
C)
1-methoxy propane
done
clear
D)
2-methoxy propane
done
clear
View Answer play_arrow
question_answer 87) \[B{{e}^{3+}}\]In this reaction, the end product C is
A)
salicylaldehyde
done
clear
B)
salicylic acid
done
clear
C)
phenyl acetate
done
clear
D)
aspirin
done
clear
View Answer play_arrow
question_answer 88) The pH of the blood does not appreciably change by a small addition of an acid or a base because
A)
serum protein which acts as buffer
done
clear
B)
containsiron as a part of the molecule
done
clear
C)
can be easily coagulated
done
clear
D)
it is body fluid
done
clear
View Answer play_arrow
question_answer 89) Hair dye contains
A)
copper nitrate
done
clear
B)
gold chloride
done
clear
C)
silver nitrate
done
clear
D)
lead nitrate
done
clear
View Answer play_arrow
question_answer 90) A gaseous hydrocarbon has 85% carbon and vapour density of 28. The possible formula of the hydrocarbon will be
A)
\[H{{e}^{+}}\]
done
clear
B)
\[x\,c{{m}^{-1}}\]
done
clear
C)
\[4x\,c{{m}^{-1}}\]
done
clear
D)
\[\frac{x}{4}\,c{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 91) The structure of \[2x\,c{{m}^{-1}}\]is
A)
linear
done
clear
B)
planar
done
clear
C)
pyramidal
done
clear
D)
T-shaped
done
clear
View Answer play_arrow
question_answer 92) Which of the following assumptions is not correct about Langmuir adsorption isotherm?
A)
The solid surface is homogeneous and has a fixed number of adsorption sites
done
clear
B)
Every adsorption site is equivalent
done
clear
C)
The adsorbed layer is not uniform al( over the adsorbent
done
clear
D)
The adsorbed gas behaves ideally in the vapour phase
done
clear
View Answer play_arrow
question_answer 93) The half-life for the reaction,\[O=16,Cu=63,N=14\] is 24h at \[1\times {{10}^{-10}}g\].Starting with 10 g of \[1\times {{10}^{-10}}g\], how many grams of \[II<I<\text{ }III<IV\] will remain after a period of 96 h?
A)
\[II<\text{ }III<I<IV\]
done
clear
B)
\[III<IV<I<II\]
done
clear
C)
\[BaC{{l}_{2}}(s)\]
done
clear
D)
\[BaC{{l}_{2}}.2{{H}_{2}}O(s)\]
done
clear
View Answer play_arrow
question_answer 94) Ceric ammonium sulphate and potassium permanganate are used as oxidising agents in acidic medium for oxidation of ferrous ammonium sulphate to ferric-sulphate. The ratio of number of moles of eerie ammonium sulphate required per mole of ferrous ammonium sulphate to the number of potassium permanganate required per mole of ferrous ammonium sulphate, is
A)
\[-20.6\]
done
clear
B)
\[8.8\text{ }kJ\]
done
clear
C)
\[BaC{{l}_{2}}(s)+2{{H}_{2}}O\xrightarrow{{}}BaC{{l}_{2}}.2{{H}_{2}}O(s),\]
done
clear
D)
\[29.4kJ\]
done
clear
View Answer play_arrow
question_answer 95) If \[-29.4kJ\] and \[-11.8kJ\] are respective equilibrium constants for the two reactions \[38.2kJ\]\[{{10}^{o}}C\]The equilibrium constant for the reaction,\[{{25}^{o}}C\]will be
A)
\[105.3\]
done
clear
B)
\[{{25}^{o}}C\]
done
clear
C)
\[{{V}_{final}}=5{{V}_{initial}}\]
done
clear
D)
\[{{V}_{initial}}>{{V}_{final}}\]
done
clear
View Answer play_arrow
question_answer 96) A compound X undergoes tetramerisation in a given organic solvent. The vant Hoff factor is
A)
\[{{\text{V}}_{\text{final}}}\text{=4}{{\text{V}}_{\text{intial}}}\]
done
clear
B)
\[{{V}_{final}}=6{{V}_{\operatorname{int}ial}}\]
done
clear
C)
\[Ca{{F}_{2}}({{K}_{sp}}=1.7\times {{10}^{-10}})\]
done
clear
D)
\[{{10}^{-4}}\,M\,C{{a}^{2+}}+{{10}^{-4}}M{{F}^{-}}\]
done
clear
View Answer play_arrow
question_answer 97) An ideal gas expands in volume from \[{{10}^{-2}}\,M\,C{{a}^{2+}}+{{10}^{-3}}M{{F}^{-}}\] to \[2{{H}^{+}}+2{{e}^{-}}+\frac{1}{2}{{O}_{2}}\xrightarrow{{}}{{H}_{2}}O(l);{{E}^{o}}=+1.23V\]at 300 K against a constant pressure of \[F{{e}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Fe(s);\,\,{{E}^{o}}=-0.44V\]. The work done is
A)
\[\Delta G\]
done
clear
B)
\[-322\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
C)
\[-161\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
D)
\[-152\,kJ\,\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 98) For \[-76\,kJ\,mo{{r}^{-1}}\] at equilibrium \[5mL\] and p is the total pressure, the ratio \[A{{s}_{2}}{{S}_{3}}\] will be
A)
\[2Ag+2HCl+(O)\xrightarrow{{}}2AgCl+{{H}_{2}}O\]
done
clear
B)
\[N{{i}_{2}}{{O}_{2}}(s)+{{H}_{2}}O(l)+2{{e}^{-}}2NiO(s)+2O{{H}^{-}}\]
done
clear
C)
\[{{E}^{o}}=+0.40V\]
done
clear
D)
\[FeO(s)+{{H}_{2}}O(l)+2{{e}^{-}}Fe(s)+2O{{H}^{-}};\]
done
clear
View Answer play_arrow
question_answer 99) Which particle among the following will have the smallest de-Broglie wavelength, assuming that they have the same velocity?
A)
A positron
done
clear
B)
A photon
done
clear
C)
An a-particle
done
clear
D)
A neutron
done
clear
View Answer play_arrow
question_answer 100) The compressibility of a gas is less than unity at STP. Therefore,
A)
\[{{E}^{o}}=-8.87V\]
done
clear
B)
\[N{{i}_{2}}{{O}_{3}}\]
done
clear
C)
\[127\,kJ\]
done
clear
D)
\[245.11kJ\]
done
clear
View Answer play_arrow
question_answer 101) The region, which is genetically active in chromosome is
A)
euchromatin
done
clear
B)
heterochromatin
done
clear
C)
whole chromosome
done
clear
D)
heptan
done
clear
View Answer play_arrow
question_answer 102) Hurlers disease is caused by
A)
presence of amino acid tyrosine
done
clear
B)
presence of lysosome
done
clear
C)
absence of lysomes
done
clear
D)
absence of amino acid tyrosine
done
clear
View Answer play_arrow
question_answer 103) How many hydrogen, bonds are present between guanine and cytosine?
A)
4
done
clear
B)
3
done
clear
C)
2
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 104) Genetically engineered human insulin is prepared by using
A)
E. coli
done
clear
B)
Rhizopus
done
clear
C)
Pseudomonas
done
clear
D)
Saccharomyces
done
clear
View Answer play_arrow
question_answer 105) The caesin is a
A)
amino acid
done
clear
B)
protein found in muscles
done
clear
C)
protein found in milk of cow
done
clear
D)
fish protein
done
clear
View Answer play_arrow
question_answer 106) Territoriality occurs as a result of
A)
co-operation
done
clear
B)
competition
done
clear
C)
parasitism
done
clear
D)
predation
done
clear
View Answer play_arrow
question_answer 107) The origin of sympathetic nerve from
A)
thoraco - lumber region
done
clear
B)
sacral region
done
clear
C)
cervical region
done
clear
D)
9th and 11th cranial nerve
done
clear
View Answer play_arrow
question_answer 108) Gauchers disease is genetic disorder, which is associated with the abnormal metabolism of
A)
glucose
done
clear
B)
fat
done
clear
C)
protein
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 109) Fruit-fly has four linkage groups. How many chromosome will be in the somatic cell of organism?
A)
4
done
clear
B)
2
done
clear
C)
8
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 110) Book lungs are respiratory organs of
A)
Nereis and arthropods
done
clear
B)
spider
done
clear
C)
scorpion
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 111) Which one era is considered as the golden period of gaint reptiles like Dinosaurs?
A)
Mesozoic
done
clear
B)
Archaeozoic
done
clear
C)
Coenozoic
done
clear
D)
Paleozoic
done
clear
View Answer play_arrow
question_answer 112) Phagocytosis was observed by
A)
Haeckel
done
clear
B)
Matchnikoff
done
clear
C)
Strassburger
done
clear
D)
Huxley
done
clear
View Answer play_arrow
question_answer 113) Choriotis nigriceps has been listed under endangered species, due to
A)
suceptible to disease
done
clear
B)
extensive hunting
done
clear
C)
climate change and spreading desert
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 114) Birds have feathers and power of flight as they lack
A)
bone marrow, an ovary and hindlimb
done
clear
B)
bone marrow, pectoral girdle and forelimb
done
clear
C)
forelimb, an ovary and bone marrow
done
clear
D)
forelimb, pectoral girdle and pelvic girdle
done
clear
View Answer play_arrow
question_answer 115) Regeneration of lost tail of lizard is an example of
A)
morpholaxis
done
clear
B)
restructury
done
clear
C)
autolysis
done
clear
D)
autotomy
done
clear
View Answer play_arrow
question_answer 116) Sporogony of Plasmodium occurs in
A)
stomach wall of mosquito
done
clear
B)
salivary gland of mosquito
done
clear
C)
liver of human beings
done
clear
D)
RBCs of human beings
done
clear
View Answer play_arrow
question_answer 117) The branch of science which deals with the process of improvement of human race through law of heredity is called
A)
Genetics
done
clear
B)
Eugenics
done
clear
C)
Euphenics
done
clear
D)
Euthenics
done
clear
View Answer play_arrow
question_answer 118) Structural property and enzymatic activity is shown by
A)
trypsin
done
clear
B)
actin
done
clear
C)
myosin
done
clear
D)
troponin
done
clear
View Answer play_arrow
question_answer 119) Which is largest muscle in human body?
A)
Masseter
done
clear
B)
Sartorius
done
clear
C)
Stapedius
done
clear
D)
Gluteus
done
clear
View Answer play_arrow
question_answer 120) The exact cause of death of cobra bite person is
A)
inactivation of nerves
done
clear
B)
inactivation and contraction of nerve and muscle
done
clear
C)
contraction of muscle and destruction of erythrocytes
done
clear
D)
destruction of RBCs
done
clear
View Answer play_arrow
question_answer 121) Sporozoite infectious stage of Plasmodium parasite contains
A)
two nucleus and a vacuole
done
clear
B)
one nucleus and several vacuole
done
clear
C)
vacuole and other cell organelles
done
clear
D)
a nucleus
done
clear
View Answer play_arrow
question_answer 122) Organs not similar in structure and origin but perform the same function
A)
homologous organ
done
clear
B)
analogous organ
done
clear
C)
adaptive structure
done
clear
D)
variable structure
done
clear
View Answer play_arrow
question_answer 123) Chloragogen cells of earthworm are analogous to vertebrates organ
A)
lungs
done
clear
B)
liver
done
clear
C)
gut
done
clear
D)
kidney
done
clear
View Answer play_arrow
question_answer 124) What used to describe as Nissls granules in a nerve cell, are now identified as?
A)
Cell metabolites
done
clear
B)
Fat granules
done
clear
C)
Ribosomes
done
clear
D)
Mitochondria
done
clear
View Answer play_arrow
question_answer 125) Photochemical smog always contain
A)
\[{{O}_{3}}\]s
done
clear
B)
oxides of sulphur
done
clear
C)
\[C{{H}_{4}}\] and \[CO\]
done
clear
D)
phosphate
done
clear
View Answer play_arrow
question_answer 126) Belivers in spontaneous generation theory assumed that
A)
organisms arose only from other similar organisms
done
clear
B)
organism could arise only from air
done
clear
C)
organism arose from non-living material
done
clear
D)
organism always arise from air
done
clear
View Answer play_arrow
question_answer 127) Maximum number of plasmids discovered so far
A)
50 kilo base
done
clear
B)
500 kilo base
done
clear
C)
5000 kilo base
done
clear
D)
5 kilo base
done
clear
View Answer play_arrow
question_answer 128) Transfer of genes form one gene pool to another is known as
A)
genetic drift
done
clear
B)
gene flow
done
clear
C)
mutation
done
clear
D)
speciation
done
clear
View Answer play_arrow
question_answer 129) Which statement is correct for muscle contraction?
A)
Length of A-band decrease
done
clear
B)
Length of 1-band remain constant
done
clear
C)
Length of two Z-line increases
done
clear
D)
Length of H-zone decreases
done
clear
View Answer play_arrow
question_answer 130) Keratinisation of skin is prevented by
A)
retinol
done
clear
B)
thiamine
done
clear
C)
ascorbic acid
done
clear
D)
calciferol
done
clear
View Answer play_arrow
question_answer 131) Core zone, buffer zone and manipulation zone are found in
A)
Zoological Garden
done
clear
B)
National Park
done
clear
C)
Tiger Reserve and Sanctuary
done
clear
D)
Biosphere Reserve
done
clear
View Answer play_arrow
question_answer 132) Largest tiger population is found in
A)
Sunder ban National Park
done
clear
B)
Corbett national Park
done
clear
C)
Ranthambor National Park
done
clear
D)
Kanha National Park
done
clear
View Answer play_arrow
question_answer 133) A child has blood group 0 and his father is of B blood group. What will be the genotype of father?
A)
\[{{l}^{A}}{{l}^{B}}\]
done
clear
B)
\[{{l}^{B}}{{l}^{O}}\]
done
clear
C)
\[{{l}^{O}}{{l}^{A}}\]
done
clear
D)
\[{{l}^{O}}{{l}^{O}}\]
done
clear
View Answer play_arrow
question_answer 134) Spermatogenesis is under the regulatory influence of
A)
STH
done
clear
B)
LH
done
clear
C)
FSH
done
clear
D)
ADH
done
clear
View Answer play_arrow
question_answer 135) Which hormone is secreted in a woman if pregnancy has occurred?
A)
Estrogen
done
clear
B)
Progesterone
done
clear
C)
Luteinizing hormone
done
clear
D)
Chorionic gonadotropin
done
clear
View Answer play_arrow
question_answer 136) Correctly matched set of phylum, class and example is
A)
Protozoa - Mastigophora -Entamoeba
done
clear
B)
Mollusca - Bivalvia -Pinctada
done
clear
C)
Arthropoda - Diplopoda - Sclopendra
done
clear
D)
Chordata - Cyclostomata - Phrynosoma
done
clear
View Answer play_arrow
question_answer 137) Which is regarded as urinary bladder of embryo?
A)
Allantois
done
clear
B)
Chorion
done
clear
C)
Yolk sac
done
clear
D)
Amnion
done
clear
View Answer play_arrow
question_answer 138) Which of these is used to control human population?
A)
Tubectomy and vasectomy
done
clear
B)
SUCD and MTP
done
clear
C)
Estrogen and progesteron
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 139) Which is thickened to form organ of corti?
A)
Reissners membrane
done
clear
B)
Basilar membrane
done
clear
C)
Tectorial membrane
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 140) Which stage of silk worm secret silk?
A)
Adult
done
clear
B)
Larva
done
clear
C)
Coccon
done
clear
D)
Pupa
done
clear
View Answer play_arrow
question_answer 141) A cell organelle lacks its own DNA, however it is able to replicate. It is
A)
centriole
done
clear
B)
ribosome
done
clear
C)
nucleolus
done
clear
D)
peroxysomes
done
clear
View Answer play_arrow
question_answer 142) A animal cell lacks nucleus. Which one will also be absent in it?
A)
Ribosome
done
clear
B)
Chromosome
done
clear
C)
Lysosome
done
clear
D)
Centriole
done
clear
View Answer play_arrow
question_answer 143) Ageing is characterised by
A)
decline in metabolic activity
done
clear
B)
increased in metabolic activity
done
clear
C)
increased anabosism
done
clear
D)
increased catabosism
done
clear
View Answer play_arrow
question_answer 144) Honey contain
A)
glucose, fructose and lactose
done
clear
B)
glucose, galactose and insulin
done
clear
C)
dextrose, laevulose and maltose
done
clear
D)
dextrose, lactose and ribose
done
clear
View Answer play_arrow
question_answer 145) Auxetic growth is increase in
A)
cell volume only
done
clear
B)
cell number only
done
clear
C)
fatly tissue
done
clear
D)
intercellular material
done
clear
View Answer play_arrow
question_answer 146) Intelligence, memory, will power, emotions and cause (reason) are governed/regulated by
A)
medulla oblongata
done
clear
B)
cerebral hemispheres
done
clear
C)
midbrain
done
clear
D)
cerebellum
done
clear
View Answer play_arrow
question_answer 147) The drug which do not develop physiological dependence are?
A)
Sedatives
done
clear
B)
Hallucinogens
done
clear
C)
Stimulants
done
clear
D)
Opiates
done
clear
View Answer play_arrow
question_answer 148) Nucleosomes given beaded appearance to chromosomes. They help in packaging of DNA in chromosomes. A nucleosomes has
A)
about two turn of DNA
done
clear
B)
8 histone molecule (two molecule each of \[{{H}_{3}},{{H}_{4}},{{H}_{2}}A\].and \[{{H}_{2}}B\])
done
clear
C)
166 nitrogen base pairs
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 149) Chromosome are best observed at metaphase. For studying the shape best stage is
A)
metaphase
done
clear
B)
anaphase
done
clear
C)
late prophase
done
clear
D)
telophase
done
clear
View Answer play_arrow
question_answer 150) Meroblastic cleavage refer to which type of division of egg?
A)
Complete
done
clear
B)
Spiral
done
clear
C)
Incomplete
done
clear
D)
Horizontal
done
clear
View Answer play_arrow
question_answer 151) Which one set of genera are correctly matched?
A)
Chlorophyceae - Volvax, Laminaria, Fucus
done
clear
B)
Phaeophyceae - Laminaria, Chlamydomonas
done
clear
C)
Rhodophyceae - Geladuim, GracHlaria
done
clear
D)
Phaeophyceae -Laminarea, Sargassum, Ulothrix
done
clear
View Answer play_arrow
question_answer 152) Vivipary is a peculiar characteristic of
A)
mesophytes
done
clear
B)
xerophytes
done
clear
C)
mangrooves
done
clear
D)
emergan hydrophytes
done
clear
View Answer play_arrow
question_answer 153) The characteristic inflorescence features of family-Moraceae and poaceae are
A)
racemose and hypanthodium
done
clear
B)
spike and spike of spikelet
done
clear
C)
hypanthodium and spadix
done
clear
D)
hypanthodium and spike of spikelet
done
clear
View Answer play_arrow
question_answer 154) The condition in which gynoecium occupy lower position or while other flower parts are situated on or above thalamus, the flower is termed as
A)
epigynous
done
clear
B)
perigynous
done
clear
C)
hypogynous
done
clear
D)
superior
done
clear
View Answer play_arrow
question_answer 155) Which of the following organism is commonly called as pond silk?
A)
Spirogyra
done
clear
B)
Ufothnx
done
clear
C)
Ectocarpus
done
clear
D)
Sargassum
done
clear
View Answer play_arrow
question_answer 156) Aleurone layer the perpheral part of grains endosperm is rich in
A)
fat
done
clear
B)
protein
done
clear
C)
carbohydrates
done
clear
D)
auxins
done
clear
View Answer play_arrow
question_answer 157) Which of the following is not on intermediate in Krebs cycle?
A)
Citric acid
done
clear
B)
Maleic acid
done
clear
C)
Acetic acid
done
clear
D)
Succinyle coenzyme-A
done
clear
View Answer play_arrow
question_answer 158) The carrier protein are involved in
A)
active transport of ions
done
clear
B)
water transport
done
clear
C)
transport of enzymes
done
clear
D)
actives and passive transport of ions
done
clear
View Answer play_arrow
question_answer 159) Who proposed the Cohesion theory for ascent of sap hypothesis?
A)
Jolly and Dixon
done
clear
B)
Dixon and Western
done
clear
C)
Jolly and Godlenski
done
clear
D)
Dixon and Strasburger
done
clear
View Answer play_arrow
question_answer 160) Turpentile oil, a ingredient in paint and varnishes is obtained from
A)
Melia azadirachta
done
clear
B)
Pinus longifolia
done
clear
C)
Eucalyputs
done
clear
D)
Cedrus
done
clear
View Answer play_arrow
question_answer 161) The anthers in family-Solanaceae
A)
introse, monothecus
done
clear
B)
extrose, dithecus
done
clear
C)
introse, dithecus
done
clear
D)
extrose, monothecus
done
clear
View Answer play_arrow
question_answer 162) The origin centre of potato is
A)
Mexico
done
clear
B)
Panama
done
clear
C)
Brazil
done
clear
D)
Peru
done
clear
View Answer play_arrow
question_answer 163) Arrangement of three successive bases in genetic code signifies
A)
amino acids
done
clear
B)
plasmids
done
clear
C)
nucleic acids
done
clear
D)
protein, peptide, peptone
done
clear
View Answer play_arrow
question_answer 164) Respiratory roots in halophytes are positively
A)
phototropic
done
clear
B)
aerotropic
done
clear
C)
rheotropic
done
clear
D)
Geotropic
done
clear
View Answer play_arrow
question_answer 165) In their experiment, Hershey and Chase demonstrated that DNA is genetic material in
A)
TMV
done
clear
B)
E coll
done
clear
C)
\[{{T}_{2}}\]-bacteriophage
done
clear
D)
Diplococcus pneumomae
done
clear
View Answer play_arrow
question_answer 166) The trapping centre of light energy in photo system-I is
A)
\[{{P}_{700}}\]
done
clear
B)
\[{{P}_{680}}\]
done
clear
C)
\[{{P}_{630}}\]
done
clear
D)
\[{{P}_{660}}\]
done
clear
View Answer play_arrow
question_answer 167) The UV radiation from sun causes reaction that produce
A)
PAN
done
clear
B)
ozone and PAN
done
clear
C)
oxides of nitrogen
done
clear
D)
acid rain
done
clear
View Answer play_arrow
question_answer 168) Deficiency of which one element cause khaira disease in paddy?
A)
\[Mn\]
done
clear
B)
\[Ca\]
done
clear
C)
\[Zn\]
done
clear
D)
\[Cl\]
done
clear
View Answer play_arrow
question_answer 169) Which one plant growth regulator is responsible for bolting?
A)
Auxin
done
clear
B)
Gibberellin
done
clear
C)
Ethylene
done
clear
D)
Cytokinin
done
clear
View Answer play_arrow
question_answer 170) When a plant show different type of leaves, the condition is termed as/known as
A)
heterophilly
done
clear
B)
homophilly
done
clear
C)
anisophilly
done
clear
D)
isophilly
done
clear
View Answer play_arrow
question_answer 171) Which of the following are fermentation product of yeast?
A)
\[C{{O}_{2}}\] and methanol
done
clear
B)
\[C{{O}_{2}}\] and ethanol
done
clear
C)
\[C{{O}_{2}}\] and water
done
clear
D)
Ethanol and water and \[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 172) Why grafting is not possible in monocots?
A)
Due to lack of cambium and parallel venation
done
clear
B)
Due to herbaceous nature
done
clear
C)
Due to lack of cambium, which is responsible for secondary growth
done
clear
D)
Due to possession of scattered vascular bundles
done
clear
View Answer play_arrow
question_answer 173) Respiratory quotient for fatty acid is
A)
zero
done
clear
B)
one
done
clear
C)
more than one
done
clear
D)
less than one
done
clear
View Answer play_arrow
question_answer 174) The synthetic auxin hormone, used as weedicides for broad leaves weeds like chenopodium is
A)
2, 4 - dichlorophenoxy acetic acid and 2, 4. 5-7
done
clear
B)
gibberellic acid
done
clear
C)
2, 4, 5 - trichlorophenoxy acetic acid
done
clear
D)
indole - 3 -acetic acid
done
clear
View Answer play_arrow
question_answer 175) The process of multiplication of DNA from DNA is known as
A)
transcription
done
clear
B)
replication
done
clear
C)
translation
done
clear
D)
transversion
done
clear
View Answer play_arrow
question_answer 176) How many turns of Calvin cycle are required to : produce one molecule of glucose?
A)
One
done
clear
B)
Three
done
clear
C)
Six
done
clear
D)
Twelve
done
clear
View Answer play_arrow
question_answer 177) The principal cereal crop of Asian countries is
A)
wheat
done
clear
B)
sorghum
done
clear
C)
barley
done
clear
D)
paddy
done
clear
View Answer play_arrow
question_answer 178) Under which one phase/stage of all cycle, cell undergoes differentiation?
A)
\[{{G}_{0}}\]-phase
done
clear
B)
\[{{G}_{1}}\]-phase
done
clear
C)
S-phase
done
clear
D)
M-phase
done
clear
View Answer play_arrow
question_answer 179) The principle protein found in locomotory organ of cell
A)
flagellin
done
clear
B)
tubulin
done
clear
C)
fibrin
done
clear
D)
globulin
done
clear
View Answer play_arrow
question_answer 180) Which state occupy first position in coffee production?
A)
Kerala
done
clear
B)
Kernataka
done
clear
C)
Tamil Nadu
done
clear
D)
Maharashtra
done
clear
View Answer play_arrow
question_answer 181) Protonema is found is the life cycle of
A)
Spirogyra
done
clear
B)
Rhizopus
done
clear
C)
Funaria
done
clear
D)
Cynodon
done
clear
View Answer play_arrow
question_answer 182) An open collateral bundle is one in which
A)
xylem and phloem is separated by cambium
done
clear
B)
two conductive tissue lie side by side
done
clear
C)
cambium occur on outside of bundle
done
clear
D)
cambium is absent in bundle
done
clear
View Answer play_arrow
question_answer 183) Dikaryon formation is characteristic feature of
A)
Zygomycetes and Phycomycetes
done
clear
B)
Phycomycetes and Ascomycetes
done
clear
C)
Basidiomycetes and Phycomycetes
done
clear
D)
Basidiomycetes and Ascomycetes
done
clear
View Answer play_arrow
question_answer 184) A prothallus of fern contain
A)
antheridia and archegonia on lower surface
done
clear
B)
antheridia and archegonia on upper surface
done
clear
C)
antheridia on upper surface and archegonia on lower surface
done
clear
D)
antheridia on lower surface and archegonia on upper surface
done
clear
View Answer play_arrow
question_answer 185) Grassland with scattered trees is a type of
A)
deciduous forest
done
clear
B)
savanna (tropical)
done
clear
C)
prairies
done
clear
D)
steppes
done
clear
View Answer play_arrow
question_answer 186) Highest number of antibiotics are produced by
A)
Bacillus
done
clear
B)
PenicHlium
done
clear
C)
Streptomyces
done
clear
D)
Cephalospohum
done
clear
View Answer play_arrow
question_answer 187) In mitochondria, enzyme cytochrome oxidase is present in
A)
matrix
done
clear
B)
outer membrane
done
clear
C)
perimitochondrial matrix
done
clear
D)
inner membrane
done
clear
View Answer play_arrow
question_answer 188) Which of the following genetically engineered bacterium is utilised for cleaning of marine oil slicks?
A)
Pseudomonas putida
done
clear
B)
Rhizoctonia soiani
done
clear
C)
Pseudomonas synngae
done
clear
D)
Nitrosomonas
done
clear
View Answer play_arrow
question_answer 189) Nucleotides consist of
A)
purine, sugar and phosphate
done
clear
B)
nucleoside and phosphate
done
clear
C)
pyrimidine sugar and phosphate
done
clear
D)
nucleoside and nitrogen base
done
clear
View Answer play_arrow
question_answer 190) Which of following floral parts form pericarp after fertilisation?
A)
Outer integument
done
clear
B)
inner integument
done
clear
C)
Ovary wall
done
clear
D)
Nucellus
done
clear
View Answer play_arrow
question_answer 191) In case of incomplete dominance, what will be the phenotypic ratio of Pa-generation?
A)
\[3:1\]
done
clear
B)
\[1:1:1:1\]
done
clear
C)
\[2:2\]
done
clear
D)
\[1:2:1\]
done
clear
View Answer play_arrow
question_answer 192) Who coined the term cistron?
A)
Benzer
done
clear
B)
Khorana
done
clear
C)
Sutton
done
clear
D)
Muller
done
clear
View Answer play_arrow
question_answer 193) Tonoplast is a membrane which surrounds
A)
mitochondria
done
clear
B)
vacuole
done
clear
C)
cytoplasm
done
clear
D)
ribosome
done
clear
View Answer play_arrow
question_answer 194) Sulphur containing amino acids are
A)
tryptophan, glutamic acid and aspartic acid
done
clear
B)
citrultine, methionine and glumeric acid
done
clear
C)
cysteine, cystine and methionine
done
clear
D)
cystine, lysine and valine
done
clear
View Answer play_arrow
question_answer 195) Which is correct sequence of code transfer involved in formation of polypeptide?
A)
DNA \[\to \] mRNA \[\to \] t RNA \[\to \] Amino acid
done
clear
B)
mRNA \[\to \] t RNA \[\to \] DNA \[\to \] Amino acid
done
clear
C)
t RNA \[\to \]DNA \[\to \] mRNA \[\to \] Amino acid
done
clear
D)
DNA \[\to \] t -RNA \[\to \] t -RNA \[\to \]mRNA
done
clear
View Answer play_arrow
question_answer 196) The conservation of endemic varieties of crop is must because
A)
these are high yielding and nutritious
done
clear
B)
we should conserve all living being of the past for our knowledge
done
clear
C)
it is mandatory
done
clear
D)
these are source of gene for genetic diversity
done
clear
View Answer play_arrow
question_answer 197) Which one of the following belong to the same category?
A)
Coconut, almond and mango
done
clear
B)
Beetle nut, chesnut and coconut
done
clear
C)
Tomato, orange and coconut
done
clear
D)
Chesnut, coconut and cashew nut
done
clear
View Answer play_arrow
question_answer 198) Which cycle is directly driven by solar radiation?
A)
Carbon
done
clear
B)
Water
done
clear
C)
Nitrogen
done
clear
D)
Phosphorus
done
clear
View Answer play_arrow
question_answer 199) Which part of embryo comes out first during seed germination
A)
Epicotyl
done
clear
B)
Hypocotyl
done
clear
C)
Radicle
done
clear
D)
Plumule
done
clear
View Answer play_arrow
question_answer 200) The cell cultured in vitro give rise to complete plants. This ability of plant cell is known as
A)
regeneration
done
clear
B)
growth
done
clear
C)
development
done
clear
D)
totipotency
done
clear
View Answer play_arrow
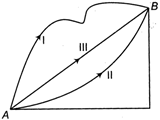
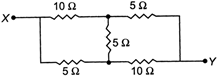
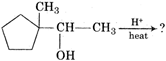 Consider the above written reaction. The product is
Consider the above written reaction. The product is
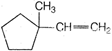
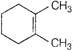
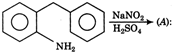 Product of this reaction is
Product of this reaction is