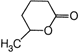question_answer 1)
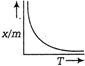
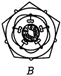
A uniform rod of copper is rotating about a axis passing through centre of mass and perpendicular to the length of the rod as shown in the diagram. Its temperature is increased by \[\frac{2{{\eta }^{2}}}{g{{a}^{2}}}\]. \[\frac{{{\eta }^{2}}}{g{{a}^{2}}}\] is coefficient of linear expansion of copper. New angular speed of the copper is
A)
\[\frac{{{\eta }^{2}}}{2g{{a}^{2}}}\]
done
clear
B)
\[\frac{{{\eta }^{2}}}{4g{{a}^{2}}}\]
done
clear
C)
\[{{t}_{1}}\]
done
clear
D)
\[{{t}_{2}}\]
done
clear
View Answer play_arrow
question_answer 2)
The Boolean expression for output in the following circuit is
A)
\[2g({{t}_{1}}+{{t}_{2}})\]
done
clear
B)
\[\frac{g}{4}{{({{t}_{1}}+{{t}_{2}})}^{2}}\]
done
clear
C)
\[g/4({{t}_{1}}{{t}_{2}})\]
done
clear
D)
\[2g{{\left( \frac{{{t}_{1}}+{{t}_{2}}}{4} \right)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 3)
Figure shows a rod of length L resting on a wall and the floor. Its lower end A is pulled towards left with a constant velocity 2v. Which of the following is the velocity of the other end B downward when the rod makes an angle (() with the horizontal?
A)
\[R/4\]
done
clear
B)
\[R/2\]
done
clear
C)
\[R/8\]
done
clear
D)
\[\left\{ \frac{{{(1+e)}^{2}}}{1+{{e}^{2}}} \right\}\sqrt{\frac{gh}{2}}\]
done
clear
View Answer play_arrow
question_answer 4)
Two circular coils can be arranged in any of the three situations shown in the figure. Their mutual inductance will be
A)
maximum in situation (ii)
done
clear
B)
maximum in situation (i)
done
clear
C)
maximum in situation (iii)
done
clear
D)
the same in all situations
done
clear
View Answer play_arrow
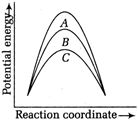
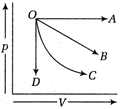
question_answer 5)
The temperature-entropy diagram of a reversible engine cycle is given in the figure. Its efficiency is
A)
\[\frac{1+{{e}^{2}}}{(1+{{e}^{2}})}\sqrt{\frac{gh}{2}}\]
done
clear
B)
\[\left\{ \frac{{{(1-e)}^{2}}}{1-{{e}^{2}}} \right\}\sqrt{\frac{gh}{2}}\]
done
clear
C)
\[\left\{ \frac{{{(1+e)}^{2}}}{1-{{e}^{2}}} \right\}\sqrt{\frac{gh}{2}}\]
done
clear
D)
\[4\Omega \]
done
clear
View Answer play_arrow
question_answer 6)
A fish sees the smiling face of a scuba diver through a bubble of air between them, as shown compared to the face of the diver the image seen by the fish will be
A)
smaller and inverted
done
clear
B)
smaller and erect
done
clear
C)
larger and erect
done
clear
D)
can be either above depending on the distance of the diver
done
clear
View Answer play_arrow
question_answer 7)
Consider the vessel shown in the adjacent diagram water being poured in the vessel at a constant rate \[2A\] There is a small hole of area a at the bottom of the vessel. The maximum level of water in the vessel is given by
A)
\[4A\]
done
clear
B)
\[3A\]
done
clear
C)
\[1A\]
done
clear
D)
\[t=0\]
done
clear
View Answer play_arrow
question_answer 8) A telescope has an objective lens of focal length 150 cm and an eyepiece with focal length 1.5 cm. If this telescope is used to see a 50 m tall building at a distance of 1.5 km, what is the height of the image of the building formed by the objective lens?
A)
10 cm
done
clear
B)
5cm
done
clear
C)
20 cm
done
clear
D)
15 cm
done
clear
View Answer play_arrow
question_answer 9) A body projected vertically upwards crosses a point twice in its journey at a height A first after \[v=4000m/s\] and \[2A\,\,\sin \,4\pi (x-200t)\] seconds. Maximum height reached by the body is
A)
\[2A\,\,\cos \,5\pi (x-400t)\]
done
clear
B)
\[A\,\,sin\,5\pi (x-400t)\]
done
clear
C)
\[A\,\,\cos \,5\pi (x-400t)\]
done
clear
D)
\[12.25V\]
done
clear
View Answer play_arrow
question_answer 10)
Six equal resistance each of R are joined to form a net word k K as shown in the figure. The resistance between any two corners is
A)
\[10.25V\]
done
clear
B)
\[11.25V\]
done
clear
C)
\[9.25V\]
done
clear
D)
R
done
clear
View Answer play_arrow
question_answer 11)
A spherical ball falls freely from a height h on to a floor having coefficient of restitution. The average speed of the ball is
A)
\[{{I}_{0}}\]
done
clear
B)
\[4{{l}_{0}}\]
done
clear
C)
\[3{{l}_{0}}\]
done
clear
D)
\[\frac{{{l}_{0}}}{2}\]
done
clear
View Answer play_arrow
question_answer 12)
In the adjoining circuit the current passing through \[2{{l}_{0}}\] resistance is
A)
\[{{I}_{0}}\]
done
clear
B)
\[2{{l}_{0}}{{\cos }^{2}}\left( \frac{\pi y}{\beta } \right)\]
done
clear
C)
\[{{l}_{0}}{{\cos }^{2}}\left( \frac{\pi y}{\beta } \right)\]
done
clear
D)
\[\frac{{{l}_{0}}}{2}{{\cos }^{2}}\left( \frac{2\pi y}{\beta } \right)\]
done
clear
View Answer play_arrow
question_answer 13)
For the wave shown in figure, if its position shown is at\[4{{l}_{0}}{{\cos }^{2}}\left( \frac{\pi y}{\beta } \right)\]. Then the equation of the wave is [when speed of wave, \[{{C}_{1}}\]]
A)
\[{{C}_{2}}\]
done
clear
B)
\[\frac{{{C}_{1}}}{{{C}_{2}}}\]
done
clear
C)
\[\frac{a}{b}\]
done
clear
D)
\[\frac{2a}{b}\]
done
clear
View Answer play_arrow
question_answer 14) A carrier wave of peak voltage 15V is used to transmit the message signal. The peak value of the modulating signal in order to have a modulation index of 75% is
A)
\[{{\left( \frac{a}{b} \right)}^{2}}\]
done
clear
B)
\[{{\left( \frac{a}{b} \right)}^{3}}\]
done
clear
C)
\[2\pi \sqrt{\frac{{{a}^{2}}}{3G\lambda }}\]
done
clear
D)
\[\pi \sqrt{\frac{{{a}^{2}}}{2G\lambda }}\]
done
clear
View Answer play_arrow
question_answer 15)
A narrow tube is bent as shown in the adjacent diagram. The radius of the tube is P and Q are small holes. Source placed at P generates a wave of intensity \[2\pi \sqrt{\frac{{{a}^{2}}}{2G\lambda }}\].The maximum intensity produced at Q is given by
A)
\[2\pi \sqrt{\frac{2{{a}^{2}}}{G\lambda }}\]
done
clear
B)
\[\frac{\sqrt{2}}{\pi }\times \frac{1}{\sqrt{15LC}}\]
done
clear
C)
\[\frac{1}{\sqrt{2}}\pi \times \frac{1}{\sqrt{15LC}}\]
done
clear
D)
\[\frac{2\sqrt{2}}{\pi }\times \frac{1}{\sqrt{15LC}}\]
done
clear
View Answer play_arrow
question_answer 16) In Youngs double slit experiment, let \[\frac{\pi }{2}\times \frac{1}{\sqrt{15LC}}\] be the intensity of the central bright fringe and P be the fringe width. The intensity of a distance Y from the central bright fringe will be
A)
\[\mu \]
done
clear
B)
\[\frac{15\lambda }{(\mu -1)}\]
done
clear
C)
\[15(\mu -1)\lambda \]
done
clear
D)
\[25(\mu -1)\lambda \]
done
clear
View Answer play_arrow
question_answer 17) Capacity of a spherical capacitor is \[\frac{25\lambda }{(\mu -1)}\], when inner sphere is charged and outer sphere is earthed and \[{{v}_{P}}=0,\,\,{{v}_{Q}}=v\] when inner sphere is earthed and outer sphere is charged. Then \[{{v}_{P}}=v,\,\,{{v}_{Q}}=0\] is (Assume a = radius of inner sphere and b = radius of outer sphere)
A)
\[{{v}_{P}}={{v}_{Q}}=0\]
done
clear
B)
\[{{v}_{P}}=0,{{v}_{Q}}=2v\]
done
clear
C)
\[\eta \]
done
clear
D)
\[\lambda \]
done
clear
View Answer play_arrow
question_answer 18)
There is an infinitely long fixed rod of linear mass density X. A small mass m is revolving around the rod at distance a from the rod with uniform speed due the gravitational pull of the rod. The time period of revolution of m is
A)
\[\frac{2\eta }{\lambda }[1-{{e}^{-\lambda t}}]\]
done
clear
B)
\[\frac{\eta }{2\lambda }[1-{{e}^{-\lambda t}}]\]
done
clear
C)
\[\frac{\eta }{{{\lambda }^{2}}}[1-{{e}^{-{{\lambda }^{2}}t}}]\]
done
clear
D)
\[\frac{\eta }{\lambda }[1-{{e}^{-\lambda t}}]\]
done
clear
View Answer play_arrow
question_answer 19)
Consider the circuit shown the frequency of oscillation is
A)
\[{{\gamma }_{1}}\]
done
clear
B)
\[{{\gamma }_{2}}\]
done
clear
C)
\[\frac{{{\gamma }_{1}}-{{\gamma }_{2}}}{3}+\frac{\alpha }{3}\]
done
clear
D)
_ \[\frac{{{\gamma }_{1}}-{{\gamma }_{2}}}{2}+\alpha \]
done
clear
View Answer play_arrow
question_answer 20) In a baptism experiments, 5th dark fringe is obtained at a point. If a thin transparent film is placed in the path of one of waves, then 7th bright fringe is obtained at the same point. The thickness of the film in terms of wavelength A, and refractive index \[\frac{{{\gamma }_{1}}-{{\gamma }_{2}}}{3}+3\alpha \] will be
A)
\[\frac{{{\gamma }_{1}}-{{\gamma }_{2}}}{3}+\alpha \]
done
clear
B)
\[{{E}_{I}}>{{E}_{II}}>{{E}_{III}}>{{E}_{IV}}>{{E}_{V}}\]
done
clear
C)
\[{{E}_{I}}>{{E}_{III}}={{E}_{V}}\,and\,\,\,{{E}_{II}}>{{E}_{IV}}\]
done
clear
D)
\[{{E}_{II}}={{E}_{IV}}={{E}_{V}}\,and\,\,\,{{E}_{I}}>{{E}_{III}}\]
done
clear
View Answer play_arrow
question_answer 21)
In the following circuit, all the bulbs are identical. The bulb(s) glowing brightest(s) is/are
A)
2, 3 and 4
done
clear
B)
5 and 6
done
clear
C)
1
done
clear
D)
1 and 2
done
clear
View Answer play_arrow
question_answer 22)
A wheel is rolling on a plane road. The linear velocity of its centre of mass is v. Then velocities of the point P and Q on the circumference of the wheel relative to road will be
A)
\[{{E}_{I}}={{E}_{II}}={{E}_{III}}\,and\,\,\,{{E}_{IV}}>{{E}_{V}}\]
done
clear
B)
\[\frac{Mgx}{4}\]
done
clear
C)
\[Mgx\]
done
clear
D)
\[\frac{Mgx}{2}\]
done
clear
View Answer play_arrow
question_answer 23) An unstable element is produced in a nuclear reaction of a constant rate \[\frac{Mgx}{3}\]. Its disintegration constant is \[\eta \]. Find the number of nuclei after time t, if initially it was zero.
A)
\[2mg\,[1+{{(\eta /L)}^{2}}]\]
done
clear
B)
\[mg\,[1+{{(\eta /L)}^{2}}]\]
done
clear
C)
\[\frac{mg}{2}\,[1+{{(\eta /L)}^{2}}]\]
done
clear
D)
\[4mg\,[1+{{(\eta /L)}^{2}}]\]
done
clear
View Answer play_arrow
question_answer 24) The coefficient of apparent expansion of a liquid when determined using two different vessels P and Q are \[\frac{100}{3}cm\]and \[100cm\] respectively. If the coefficient of linear expansion of the vessel P is a, the coefficient of linear expansion of the vessel Q is
A)
\[\frac{200}{3}cm\]
done
clear
B)
\[\frac{50}{3}cm\]
done
clear
C)
\[T=2{{T}_{0}}+\eta {{V}^{2}}\]
done
clear
D)
\[\eta \]
done
clear
View Answer play_arrow
question_answer 25)
Variation of electric potential V as a function of distance x through five regions on X-axis. Which of the following is true regarding electric field E in these regions
A)
\[\sqrt{2}R\sqrt{\eta {{T}_{0}}}\]
done
clear
B)
\[2R\sqrt{\eta {{T}_{0}}}\]
done
clear
C)
\[2\sqrt{2}R\sqrt{\eta {{T}_{0}}}\]
done
clear
D)
\[R\sqrt{\eta {{T}_{0}}}\]
done
clear
View Answer play_arrow
question_answer 26) A metal wire of length L, area of cross-section A and Youngs modulus Y is stretched by a variable force F such that F is always slightly greater than the elastic forces of resistance in the wire. When the elongation of the wire is x.
A)
\[8H\]
done
clear
B)
\[14H\]
done
clear
C)
\[7H\]
done
clear
D)
\[21H\]
done
clear
View Answer play_arrow
question_answer 27)
A simple pendulum of length L and mass m of the bob is vibrating with an amplitude \[\text{n}=4\]. Then the maximum tension in the string is
A)
\[\text{n}=1\]
done
clear
B)
\[2.5\text{ }m/s\]
done
clear
C)
\[2.07\text{ }m/s\]
done
clear
D)
\[4.08\text{ }m/s\]
done
clear
View Answer play_arrow
question_answer 28) The power of a lens, a short sighted uses is -3D. Find the maximum distance of an object which he/she can see without spectacles.
A)
\[5.08\text{ }m/s\]
done
clear
B)
\[{{\mu }_{s}}=0.4\]
done
clear
C)
\[g=10m/{{s}^{2}}\]
done
clear
D)
\[0.5m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 29) During expansion of one mole of an ideal gas temperature and volume are associated as \[1m/{{s}^{2}}\], where To and \[0.25m/{{s}^{2}}\] are positive constants. Find the minimum attainable pressure of the gas.
A)
\[0.75m/{{s}^{2}}\]
done
clear
B)
\[\Delta T\]
done
clear
C)
\[\alpha \]
done
clear
D)
\[2\omega [1-2\alpha \Delta T]\]
done
clear
View Answer play_arrow
question_answer 30) A large bubble rises from the bottom of a lake to the surface, its radius doubles. One atmospheric pressure is equal to that of a column of water of height 2H. The depth of the lake is
A)
\[\omega [1-\alpha \Delta T]\]
done
clear
B)
\[\omega [1+2\alpha \Delta T]\]
done
clear
C)
\[\omega [1+2\alpha \Delta T]\]
done
clear
D)
\[P+Q+R+S\]
done
clear
View Answer play_arrow
question_answer 31) The recoil speed of a hydrogen atom after it emits a photon in going from \[\overline{P}\overline{Q}\overline{R}\overline{S}\]to \[(P+Q)(R+S)\] state is
A)
\[\overline{PQRS}\]
done
clear
B)
\[2v\,\tan \phi \]
done
clear
C)
\[v\,\tan \phi \]
done
clear
D)
\[v\,\cot \phi \]
done
clear
View Answer play_arrow
question_answer 32)
Consider two blocks P and Q. A force F is applied on the block P and the block Q is at rest. The coefficient of static friction between P and Q is \[2v\,\cot \phi \]. Find acceleration of each block if \[20%\] weight of P and Q are 100 N and 300N, respectively. F = 20N.
A)
\[33%\]
done
clear
B)
\[43%\]
done
clear
C)
\[50%\]
done
clear
D)
\[\eta \,{{m}^{3}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
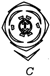
question_answer 33)
The linear charge density of four quadrants or aring of radius a is as shown. Electric field at centre will be
A)
\[\frac{2{{\eta }^{2}}}{g{{a}^{2}}}\]
done
clear
B)
zero
done
clear
C)
\[\frac{{{\eta }^{2}}}{g{{a}^{2}}}\]
done
clear
D)
\[\frac{{{\eta }^{2}}}{2g{{a}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 34)
A man is moving down an incline plane with speed u. He meets rain with the same speed but horizontally.
A)
\[\frac{{{\eta }^{2}}}{4g{{a}^{2}}}\]
done
clear
B)
\[{{t}_{1}}\]
done
clear
C)
\[{{t}_{2}}\]
done
clear
D)
\[2g({{t}_{1}}+{{t}_{2}})\]
done
clear
View Answer play_arrow
question_answer 35)
Consider the given circuit, the reading of voltmeter \[\frac{g}{4}{{({{t}_{1}}+{{t}_{2}})}^{2}}\] and \[g/4({{t}_{1}}{{t}_{2}})\] are 500V each. The reading of the voltmeter V. and ammeter a respectively are
A)
\[2g{{\left( \frac{{{t}_{1}}+{{t}_{2}}}{4} \right)}^{2}}\]
done
clear
B)
\[R/4\]
done
clear
C)
\[R/2\]
done
clear
D)
\[R/8\]
done
clear
View Answer play_arrow
question_answer 36)
The spectra of radiation emitted by two distant stars are as shown below. The ratio of the surface temperature of star A to that of star B, \[\left\{ \frac{{{(1+e)}^{2}}}{1+{{e}^{2}}} \right\}\sqrt{\frac{gh}{2}}\] is approximately
A)
\[\frac{1+{{e}^{2}}}{(1+{{e}^{2}})}\sqrt{\frac{gh}{2}}\]
done
clear
B)
\[\left\{ \frac{{{(1-e)}^{2}}}{1-{{e}^{2}}} \right\}\sqrt{\frac{gh}{2}}\]
done
clear
C)
\[\left\{ \frac{{{(1+e)}^{2}}}{1-{{e}^{2}}} \right\}\sqrt{\frac{gh}{2}}\]
done
clear
D)
\[4\Omega \]
done
clear
View Answer play_arrow
question_answer 37) In a transistor amplifier \[2A\] \[4A\] and input resistance of the transistor is \[3A\] What is the power amplification?
A)
\[1A\]
done
clear
B)
\[t=0\]
done
clear
C)
\[v=4000m/s\]
done
clear
D)
\[2A\,\,\sin \,4\pi (x-200t)\]
done
clear
View Answer play_arrow
question_answer 38)
For the toppling of the shown figure regular hexagon. The coefficient of friction must be
A)
\[2A\,\,\cos \,5\pi (x-400t)\]
done
clear
B)
\[A\,\,sin\,5\pi (x-400t)\]
done
clear
C)
\[A\,\,\cos \,5\pi (x-400t)\]
done
clear
D)
\[12.25V\]
done
clear
View Answer play_arrow
question_answer 39) An iron rod of \[10.25V\] cross-sectional area is subjected to a magnetising field of\[11.25V\]. If the susceptibility of iron is 499, the magnetic flux produced is
A)
\[9.25V\]
done
clear
B)
\[{{I}_{0}}\]
done
clear
C)
\[4{{l}_{0}}\]
done
clear
D)
\[3{{l}_{0}}\]
done
clear
View Answer play_arrow
question_answer 40)
Consider two block system \[\frac{{{l}_{0}}}{2}\] and \[2{{l}_{0}}\]. If \[{{I}_{0}}\] is removed suddenly, find the angular frequency and amplitude of \[2{{l}_{0}}{{\cos }^{2}}\left( \frac{\pi y}{\beta } \right)\].
A)
\[{{l}_{0}}{{\cos }^{2}}\left( \frac{\pi y}{\beta } \right)\]and \[\frac{{{l}_{0}}}{2}{{\cos }^{2}}\left( \frac{2\pi y}{\beta } \right)\]
done
clear
B)
\[4{{l}_{0}}{{\cos }^{2}}\left( \frac{\pi y}{\beta } \right)\] and \[{{C}_{1}}\]
done
clear
C)
\[{{C}_{2}}\] and \[\frac{{{C}_{1}}}{{{C}_{2}}}\]
done
clear
D)
\[\frac{a}{b}\] and \[\frac{2a}{b}\]
done
clear
View Answer play_arrow
question_answer 41) A proton is moving in a region of uniform magnetic field has a velocity \[{{\left( \frac{a}{b} \right)}^{2}}\]and an acceleration \[{{\left( \frac{a}{b} \right)}^{3}}\]. Then,
A)
\[2\pi \sqrt{\frac{{{a}^{2}}}{3G\lambda }}\]
done
clear
B)
\[\pi \sqrt{\frac{{{a}^{2}}}{2G\lambda }}\]
done
clear
C)
\[2\pi \sqrt{\frac{{{a}^{2}}}{2G\lambda }}\]
done
clear
D)
\[2\pi \sqrt{\frac{2{{a}^{2}}}{G\lambda }}\]
done
clear
View Answer play_arrow
question_answer 42) Two vectors P and Q are related as \[\frac{\sqrt{2}}{\pi }\times \frac{1}{\sqrt{15LC}}\], then the value of \[\frac{1}{\sqrt{2}}\pi \times \frac{1}{\sqrt{15LC}}\] is
A)
\[\frac{2\sqrt{2}}{\pi }\times \frac{1}{\sqrt{15LC}}\]
done
clear
B)
\[\frac{\pi }{2}\times \frac{1}{\sqrt{15LC}}\]
done
clear
C)
\[\mu \]
done
clear
D)
\[\frac{15\lambda }{(\mu -1)}\]
done
clear
View Answer play_arrow
question_answer 43) 2N division on main scale of a vernier calliper coincide with \[15(\mu -1)\lambda \] division of the vernier scale if each division on main scale is of b units, least count of instrument is
A)
\[25(\mu -1)\lambda \]
done
clear
B)
\[\frac{25\lambda }{(\mu -1)}\]
done
clear
C)
\[{{v}_{P}}=0,\,\,{{v}_{Q}}=v\]
done
clear
D)
\[{{v}_{P}}=v,\,\,{{v}_{Q}}=0\]
done
clear
View Answer play_arrow
question_answer 44) Consider the reaction \[{{v}_{P}}={{v}_{Q}}=0\]. If \[{{v}_{P}}=0,{{v}_{Q}}=2v\]and \[\eta \]. The energy Q released (in MeV) in this fusion reaction is
A)
\[\lambda \]
done
clear
B)
\[\frac{2\eta }{\lambda }[1-{{e}^{-\lambda t}}]\]
done
clear
C)
\[\frac{\eta }{2\lambda }[1-{{e}^{-\lambda t}}]\]
done
clear
D)
\[\frac{\eta }{{{\lambda }^{2}}}[1-{{e}^{-{{\lambda }^{2}}t}}]\]
done
clear
View Answer play_arrow
question_answer 45) An experiment conducted to determine Young s modulus , \[\frac{\eta }{\lambda }[1-{{e}^{-\lambda t}}]\] , where Y = Youngs modulus, T = time, t = torque and 7 = length. Then, the value of \[{{\gamma }_{1}}\]is
A)
1
done
clear
B)
0
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 46)
A block of mass M is pulled by a constant power P is placed on a rough horizontal plane. The frictional coefficient between block and surface is \[{{\gamma }_{2}}\] Find the maximum velocity of the block.
A)
\[\frac{{{\gamma }_{1}}-{{\gamma }_{2}}}{3}+\frac{\alpha }{3}\]
done
clear
B)
\[\frac{{{\gamma }_{1}}-{{\gamma }_{2}}}{2}+\alpha \]
done
clear
C)
\[\frac{{{\gamma }_{1}}-{{\gamma }_{2}}}{3}+3\alpha \]
done
clear
D)
\[\frac{{{\gamma }_{1}}-{{\gamma }_{2}}}{3}+\alpha \]
done
clear
View Answer play_arrow
question_answer 47) Radius of a circular current carrying coil is a. At a distance no. from centre of the coil on its axis, intensity of magnetic field is found to be \[{{E}_{I}}>{{E}_{II}}>{{E}_{III}}>{{E}_{IV}}>{{E}_{V}}\] times that at the centre, then
A)
\[{{E}_{I}}>{{E}_{III}}={{E}_{V}}\,and\,\,\,{{E}_{II}}>{{E}_{IV}}\]
done
clear
B)
\[{{E}_{II}}={{E}_{IV}}={{E}_{V}}\,and\,\,\,{{E}_{I}}>{{E}_{III}}\]
done
clear
C)
\[{{E}_{I}}={{E}_{II}}={{E}_{III}}\,and\,\,\,{{E}_{IV}}>{{E}_{V}}\]
done
clear
D)
\[\frac{Mgx}{4}\]
done
clear
View Answer play_arrow
question_answer 48)
In the figure, a small block P is released from rest when the spring is at its natural length. For the disc Q, of mass \[Mgx\] to leave contact with the ground at same stage, the minimum mass of P must be
A)
\[\frac{Mgx}{2}\]
done
clear
B)
\[\frac{Mgx}{3}\]
done
clear
C)
\[\eta \]
done
clear
D)
function of \[2mg\,[1+{{(\eta /L)}^{2}}]\]and spring constant
done
clear
View Answer play_arrow
question_answer 49) If frequency of \[mg\,[1+{{(\eta /L)}^{2}}]\] X-ray emitted from element with \[\frac{mg}{2}\,[1+{{(\eta /L)}^{2}}]\] is \[4mg\,[1+{{(\eta /L)}^{2}}]\], then frequency of , \[\frac{100}{3}cm\] X-rays emitted from element with \[100cm\] would be
A)
\[\frac{200}{3}cm\]
done
clear
B)
\[\frac{50}{3}cm\]
done
clear
C)
\[T=2{{T}_{0}}+\eta {{V}^{2}}\]
done
clear
D)
\[\eta \]
done
clear
View Answer play_arrow
question_answer 50)
A cork floating is a calm pond executes simple harmonic motion of frequency v when a wave generated by a boat passes by it. The frequency of the wave is
A)
\[\sqrt{2}R\sqrt{\eta {{T}_{0}}}\]
done
clear
B)
\[2R\sqrt{\eta {{T}_{0}}}\]
done
clear
C)
\[2\sqrt{2}R\sqrt{\eta {{T}_{0}}}\]
done
clear
D)
v
done
clear
View Answer play_arrow
question_answer 51)
Consider the following statements. If the van der Walls parameters of two gases are given as \[2A+2B2C+2D;\,\,\,\,{{E}^{o}}=y\,volt,\,\,\,\,{{K}_{eq}}={{K}_{2}}\] \[x=y,{{K}_{2}}=K_{1}^{2}\] Gas X \[x=2y,{{K}_{1}}=2{{K}_{2}}\] \[x=y.{{K}_{1}}=\frac{1}{{{K}_{2}}}\] Gas Y \[x=y,{{K}_{1}}=K_{2}^{2}\] \[XC{{l}_{n}}\]
I. Critical volume of X > critical volume of Y II. Critical pressure of X > critical pressure of V III. Critical temperature of X > critical temperature of Y.
Which of the above statements is/are true?
A)
Only I
done
clear
B)
I and II
done
clear
C)
I, II and III
done
clear
D)
II and III
done
clear
View Answer play_arrow
A)
\[XC{{l}_{n-2}}\]
done
clear
B)
\[A>B>C\]
done
clear
C)
\[B>A>C\]
done
clear
D)
\[A>C>B\]
done
clear
View Answer play_arrow
question_answer 53) Permanent hardness is due to \[C>B>A\] and \[F{{e}_{0.93}}{{O}_{1.00}}\] of \[2C{{u}_{2}}O+C{{u}_{2}}S\xrightarrow{{}}6Cu+S{{O}_{2}}\] and \[2C{{u}_{2}}S+3{{O}_{2}}\xrightarrow{{}}2C{{u}_{2}}O+S{{O}_{2}}\] and is removed by the addition of \[2CuFe{{S}_{2}}+{{O}_{2}}\xrightarrow{{}}C{{u}_{2}}S+FeS+S{{O}_{2}}\] \[2FeS+3{{O}_{2}}\xrightarrow{{}}2FeO+2S{{O}_{2}}\] \[q=\Delta U\] If hardness is 100 ppm \[O\to D\], amount of\[O\to C\] required to soften 10 L of hard water, is
A)
\[O\to B\]
done
clear
B)
\[O\to A\]
done
clear
C)
\[A{{g}_{2}}O+{{H}_{2}}O+2{{e}^{-}}\xrightarrow{{}}2Ag+2O{{H}^{-}}\]
done
clear
D)
\[Fe(III)\]
done
clear
View Answer play_arrow
question_answer 54) Elements A, B and C have atomic numbers 19, 37 and 55 respectively. Which of the following statement is correct about them?
A)
Their ionisation potential would increase with increasing atomic number
done
clear
B)
B would have an ionisation potential between those of A and C
done
clear
C)
C would have the highest ionisation potential
done
clear
D)
B would have the highest ionisation potential
done
clear
View Answer play_arrow
question_answer 55) If wavelength of 1st line of Balmer series, is \[Fe(II)\], then wavelength of 2nd line of Balmer series is
A)
\[Mn(II)\]
done
clear
B)
\[Cr(II)\]
done
clear
C)
\[\sigma \]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}OH+HX\xrightarrow{Zn{{X}_{2}}}{{C}_{2}}{{H}_{5}}X,\]
done
clear
View Answer play_arrow
question_answer 56) Find out the change in entropy when two gram atoms of aluminium is heated from \[HCl>HBr>Hl\] to \[HBr>Hl>HCl\]. The atomic heat capacity of aluminium is given by (\[Hl>HBr>HCl~\] T cal/deg)
A)
\[Hl>HCl>HBr\]
done
clear
B)
\[C{{H}_{3}}Mgl+C{{H}_{3}}CHOHC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}Mgl+C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}MgBr+C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 57) A molecule \[C{{H}_{3}}C{{H}_{2}}MgBr+C{{H}_{3}}COC{{H}_{3}}\] contains two a, two TC-bonds and one lone pair of electron in the valence shell of A. The arrangement of lone pair as well as bond pairs, is
A)
square pyramidal
done
clear
B)
trigonal planar
done
clear
C)
linear
done
clear
D)
tetrahedral
done
clear
View Answer play_arrow
question_answer 58)
The IUPAC name of the following polyfunctional compound is
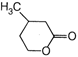
A)
2, 4-dioxocycloheptanoic acid
done
clear
B)
2,4-dioxocyclohexanoic acid
done
clear
C)
2,4-dioxocyclohexane -1- carboxylic acid
done
clear
D)
4 - formal - 2-oxocyclohexane-1-carboxytic acid
done
clear
View Answer play_arrow
question_answer 59) Which of the following buffer solution turns invalid on addition of 10 mL of \[{{25}^{o}}C\]?
A)
\[C{{H}_{3}}COOH\] having \[N{{H}_{4}}OH\] \[({{10}^{-5}})\] and \[({{10}^{-5}})\] each
done
clear
B)
\[N{{H}_{4}}OH\] having \[4.0\] and \[3.0\] \[11.0\]each
done
clear
C)
\[10.0\] having \[\overset{O}{\mathop{\overset{||}{\mathop{C{{H}_{3}}-C-C{{H}_{2}}-C{{H}_{2}}-COOH}}\,}}\,\]\[\xrightarrow[(ii)\,\,{{H}_{2}}O+{{H}^{+}}]{(i)\,\,NaB{{H}_{4}}}\] and \[\beta \] each
done
clear
D)
\[\alpha \] having\[\alpha \] \[\beta \]and \[\beta \]each
done
clear
View Answer play_arrow
question_answer 60) Consider the reaction \[\beta \] with reference to the given reaction, which one of the following is the true statement?
A)
\[{{\Delta }_{O}}\] is oxidised to \[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
B)
\[[Fe{{(CN)}_{2}}{{({{H}_{2}}O)}_{4}}]\] is oxidised to \[{{[Fe{{(CN)}_{4}}({{H}_{2}}{{O}_{2}})]}^{2-}}\]
done
clear
C)
\[{{\Delta }_{O}}(II)<{{\Delta }_{O}}(III)<{{\Delta }_{O}}(I)\] is oxidised to \[{{\Delta }_{O}}(III)<{{\Delta }_{O}}(II)<{{\Delta }_{O}}(I)\]
done
clear
D)
Zn is reduced to \[{{\Delta }_{O}}(II)<{{\Delta }_{O}}(I)<{{\Delta }_{O}}(III)\]
done
clear
View Answer play_arrow
question_answer 61) Various phenol derivatives, tincture of iodine (\[{{\Delta }_{O}}(I)<{{\Delta }_{O}}(II)<{{\Delta }_{O}}(III)\] in water/alcohol) and some dyes like methylene blue are
A)
antipyretics
done
clear
B)
analgesics
done
clear
C)
antiseptics
done
clear
D)
disinfectants
done
clear
View Answer play_arrow
question_answer 62) When hard water is passed through permutit, which ions are exchanged with \[III<I<IV<II\]and \[I<IV<II<III\]?
A)
\[\text{I}<III<II<IV\]
done
clear
B)
\[I>III>IV>II\]
done
clear
C)
\[FeC{{r}_{2}}{{O}_{4}}\xrightarrow{I}N{{a}_{2}}Cr{{O}_{4}}\xrightarrow{II}C{{r}_{2}}{{O}_{3}}\xrightarrow{III}Cr\]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 63) If the distance between \[NaOH/air\] and \[Al,\] ions in sodium chloride is y pm, the length of the edge of the unit cell is
A)
2ypm
done
clear
B)
y/2pm
done
clear
C)
y/4 pm
done
clear
D)
4ypm
done
clear
View Answer play_arrow
question_answer 64) \[NaOH/air,\Delta \] gas along with solid [B] is obtained when sodium salt is heated. A is again obtained when \[N{{a}_{2}}C{{O}_{3}}/air,\Delta \] gas is passed into aqueous solution of B. A and B respectively are
A)
\[N{{H}_{4}}Cl,\Delta \]
done
clear
B)
\[Al\]
done
clear
C)
\[N{{a}_{2}}C{{O}_{3}}/air,\Delta \]
done
clear
D)
\[{{V}_{C}}=3b\ \,equal\]
done
clear
View Answer play_arrow
question_answer 65) Identify C in the series\[=3\times 0.056=0.168d{{m}^{3}}mo{{l}^{-1}}\]\[{{p}_{C}}=\frac{a}{27{{b}^{2}}}\]
A)
\[a/d{{m}^{6}}\,bar\,mo{{l}^{-2}}\]
done
clear
B)
\[b/d{{m}^{6}}\,mo{{l}^{-2}}\]
done
clear
C)
\[6.5\]
done
clear
D)
\[0.056\]
done
clear
View Answer play_arrow
question_answer 66) A reacts with aqueous \[18.0\] solution to form B and Hz- Aqueous solution of B is heated upto 323-333K and on passing \[0.011\] into it, \[IV<V<I<III<II\] and C were formed. When C is heated upto\[V<IV<III<I<II\]. \[II>IV<III<V<I\] is formed. A, B and C respectively are
A)
\[III<IV<I<V<II\]
done
clear
B)
\[SO_{4}^{2-}\]
done
clear
C)
\[C{{l}^{-}}\]
done
clear
D)
\[C{{a}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 67) Forcarbamon, \[M{{g}^{2+}}\] Stability order will be
A)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
B)
\[CaS{{O}_{4}}+N{{a}_{2}}C{{O}_{3}}\xrightarrow{{}}CaC{{O}_{3}}+N{{a}_{2}}S{{O}_{4}}\]
done
clear
C)
\[CaC{{l}_{2}}+N{{a}_{2}}C{{O}_{3}}\xrightarrow{{}}CaC{{O}_{3}}+2NaCl\]
done
clear
D)
\[CaC{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 68) Hydrofluoric acid cannot be stored in glass vessels due to it reacts with glass to form
A)
\[N{{a}_{2}}C{{O}_{3}}\] and \[4.81\text{ }g\]
done
clear
B)
\[3.24\text{ }g\]
done
clear
C)
\[1.06\text{ }g\]
done
clear
D)
\[2.03\text{ }g\]
done
clear
View Answer play_arrow
question_answer 69)
A)
an optically inactive compound
done
clear
B)
an optically active compound
done
clear
C)
a racemic mixture
done
clear
D)
a diastereomeric mixture
done
clear
View Answer play_arrow
question_answer 70) The density of nitrogen gas prepared from air is slightly greater than that of nitrogen prepared by a chemical reaction from a compound of nitrogen because of-the presence of which of the following in aerial nitrogen?
A)
\[6500\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
Argon
done
clear
C)
Greater amount of nitrogen molecules derived from \[4814.8\overset{\text{o}}{\mathop{\text{A}}}\,\] isotope
done
clear
D)
Some nitrogen molecules analogous to \[5884.3\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 71) When rain is accompanied by a thunderstorm, the collected rain wafer will have a pH value
A)
slightly lower than that of rain water without thunderstorm
done
clear
B)
slightly higher than that when the thunderstorm is not these
done
clear
C)
uninfluenced by occurrance of thunderstorm
done
clear
D)
which depends on the amount of dust in air
done
clear
View Answer play_arrow
question_answer 72) 0.005 cm thick coating of silver is deposited on a plate of \[4728.6\overset{\text{o}}{\mathop{\text{A}}}\,\]area. The number of silver atoms deposited on the plate are (atomic mass of \[6172.8\overset{\text{o}}{\mathop{\text{A}}}\,\]and its density\[{{25}^{o}}C\])
A)
\[{{600}^{o}}C\]
done
clear
B)
\[{{C}_{p}}=5.006+0.0026T\]
done
clear
C)
\[138\text{ }cal/deg\]
done
clear
D)
\[13.75\text{ }cal/deg\]
done
clear
View Answer play_arrow
question_answer 73) The Ionic radii of group-12 metals Zn, Cd and Hg are smaller than those of group-2 metals because of Zn, Cd and Hg have
A)
10 d-electrons which have a large exchange energy
done
clear
B)
10 d-electrons which have a large radius ratio
done
clear
C)
10 d-electrons which shield the nuclear charge strongly
done
clear
D)
10 d-electrons which shield the nuclear charge poorly
done
clear
View Answer play_arrow
question_answer 74) The order of increasing freezing point of \[18.28eu\] and \[23.41\text{ }eu\] is
A)
\[A{{B}_{2}}\]
done
clear
B)
\[1.0M\text{ }HCl\]
done
clear
C)
\[100mL\]
done
clear
D)
\[0.05M\]
done
clear
View Answer play_arrow
question_answer 75) What is the correct relationship between the pH of isomolar solution of sodium oxide \[N{{H}_{3}}\], sodium sulphide \[N{{H}_{4}}Cl\], sodium selenide \[100mL\] and sodium telluride \[0.2M\]?
A)
\[0.1M\]
done
clear
B)
\[N{{H}_{4}}Cl\]
done
clear
C)
\[100mL\]
done
clear
D)
\[0.2M\]
done
clear
View Answer play_arrow
question_answer 76) Which among the following is the correct mathematical representation of Daltons law of partial pressure?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[N{{H}_{4}}Cl\]
done
clear
C)
\[100mL\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 77) The standard electrode potential \[0.15M\] for \[N{{H}_{3}}\] and \[N{{H}_{4}}Cl\] respectively are 0.94 V and -\[Zn+C{{u}^{2+}}\xrightarrow{{}}Z{{n}^{2+}}+Cu\]. The \[C{{u}^{2+}}\] value for \[Cu\] will be
A)
\[Z{{n}^{2+}}\]
done
clear
B)
\[Zn\]
done
clear
C)
\[Zn\]
done
clear
D)
\[Z{{n}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 78)
Which of the following are is electronic species? I. \[Z{{n}^{2+}}\] II. \[2-3%{{I}_{2}}\] III. \[C{{a}^{2+}}\] IV. \[M{{g}^{2+}}\]
A)
I, III and IV
done
clear
B)
I, II and IV
done
clear
C)
II, Ill and IV
done
clear
D)
I and II
done
clear
View Answer play_arrow
question_answer 79) Consider the following equation for a cell reaction, \[A{{l}^{3+}}\] \[{{H}^{+}}\] then
A)
\[N{{a}^{+}}\]
done
clear
B)
\[N{{a}^{+}}\]
done
clear
C)
\[C{{l}^{-}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 80) A solid element (symbol X) conducts electricity and forms two chlorides \[C{{O}_{2}}\] [a colourless volatile liquid) and \[N{{a}_{2}}C{{O}_{3}},NaOH\] (a colourless solid). To which one of the following groups of the periodic table does X belong?
A)
16
done
clear
B)
15
done
clear
C)
14
done
clear
D)
13
done
clear
View Answer play_arrow
question_answer 81)
If a homogeneous catalytic reaction can take place through three alternative paths as depicted below, the catalytic efficiency of A, B, C representing the relative ease would be
A)
\[N{{a}_{2}}C{{O}_{3}},N{{a}_{2}}O\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}},NaHC{{O}_{3}}\]
done
clear
C)
\[NaHC{{O}_{3}},N{{a}_{2}}C{{O}_{3}}\]
done
clear
D)
\[{{C}_{3}}{{H}_{7}}OH\xrightarrow{Conc.\,{{H}_{2}}S{{O}_{4}}}A\xrightarrow{B{{r}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 82)
In which of the properties listed below, hydrogen does not show similarity with halogens? I. Nature of the oxide II. Electropositive character III. Combination with alkali metals IV. Atomicity
A)
I and III
done
clear
B)
I and II
done
clear
C)
III and IV
done
clear
D)
Only II
done
clear
View Answer play_arrow
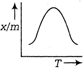
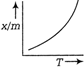
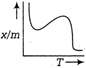
question_answer 83) Which of the following graphs represents the variation of amount of chemisorption of a gas by a solid with temperature under constant pressure condition?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 84) The composition of a sample of wustite is \[B\xrightarrow{\begin{smallmatrix} Excess\,of \\ alc.\,KOH \end{smallmatrix}}C\] What percentage of iron is present in the form of Fe(III)?
A)
75%
done
clear
B)
45%,
done
clear
C)
15%
done
clear
D)
35%
done
clear
View Answer play_arrow
question_answer 85) In the extraction of copper, the reaction which does not take place in Bessemer converter, is
A)
\[C{{H}_{3}}-C\equiv CH\]
done
clear
B)
\[\underset{OH}{\mathop{\underset{|}{\mathop{C{{H}_{3}}-C=C{{H}_{2}}}}\,}}\,\]
done
clear
C)
\[\underset{OH}{\mathop{\underset{|}{\mathop{C{{H}_{3}}-CH=\underset{OH}{\mathop{\underset{|}{\mathop{C{{H}_{2}}}}\,}}\,}}\,}}\,\]
done
clear
D)
\[\underset{N{{H}_{2}}N{{H}_{2}}}{\mathop{\underset{||}{\mathop{C{{H}_{3}}-CH-C{{H}_{2}}}}\,}}\,\]
done
clear
View Answer play_arrow
question_answer 86)
For which of the following process, \[NaOH\]?
A)
\[C{{O}_{2}}\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
C)
\[{{1200}^{o}}C\]
done
clear
D)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 87) In the chemical reaction,\[Al,NaAl{{O}_{2}},Al{{(OH)}_{3}}\]
A)
electrons are reduced
done
clear
B)
water is oxidized
done
clear
C)
silver is reduced
done
clear
D)
silver is oxidized
done
clear
View Answer play_arrow
question_answer 88) A complex of a certain metal ion has a magnetic moment of 4.90 BM, another complex of the same metal in the same oxidation state has a zero magnetic moment. The central metal ion could be
A)
\[Al,Al{{(OH)}_{3}},AlC{{l}_{3}}\]
done
clear
B)
\[Al,AlC{{l}_{3}},NaAl{{O}_{2}}\]
done
clear
C)
\[Zn,N{{a}_{2}}Zn{{O}_{2}},Al{{(OH)}_{2}}\]
done
clear
D)
\[\begin{matrix} {{R}_{3}}\overline{C} \\ P \\ \end{matrix}\,\,\,\,\,\,\,\,\,\begin{matrix} {{R}_{2}}\overline{C}H \\ Q \\ \end{matrix}\,\,\,\,\,\,\,\,\,\begin{matrix} \overline{C}{{H}_{3}} \\ R \\ \end{matrix}\,\,\,\,\,\,\,\,\,\,\begin{matrix} R-\overline{C}{{H}_{2}} \\ S \\ \end{matrix}\]
done
clear
View Answer play_arrow
question_answer 89) The electrophile, E+ attacks the benzene ring to generate the intermediate a-complex. Of the following, which \[P>Q>S>R\]-complex is of lowest energy?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 90) For the reaction\[R>S>Q>P\] the order of reactivity is
A)
\[Q>R>P>S\]
done
clear
B)
\[R>Q>S>P\]
done
clear
C)
\[N{{a}_{2}}Si{{O}_{3}}\]
done
clear
D)
\[{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 91) Which of the following are the starting materials for the Grignards synthesis of tert butyl alcohol?
A)
\[Si{{F}_{2}}\]
done
clear
B)
\[N{{a}_{2}}Si{{F}_{6}}\]
done
clear
C)
\[N{{a}_{4}}[Si{{F}_{6}}]\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 92) At \[{{N}^{15}}\], the dissociation constants of \[{{O}_{2}}\] and \[0.5{{m}^{2}}\] in aqueous solution are almost same \[Ag=108\]. If pH of some acetic \[=7.9\text{ }ge{{m}^{-3}}\] acid solution is 3. The pH of solution of \[1.1\times {{10}^{15}}\] of same concentration at the same temperature would be
A)
\[1.1\times {{10}^{24}}\]
done
clear
B)
\[1.1\times {{10}^{23}}\]
done
clear
C)
\[1.1\times {{10}^{32}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}OH,B{{a}_{3}}(P{{O}_{4}}),N{{a}_{2}}S{{O}_{4}},LiP{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 93) End product of this conversion,\[KCl\] \[B{{a}_{3}}{{(P{{O}_{4}})}_{2}}<L{{i}_{3}}P{{O}_{4}}<N{{a}_{2}}S{{O}_{4}}<KCl<{{C}_{2}}{{H}_{5}}OH\]is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 94) Aniline when diazotised in cold and then treated with dimethyl aniline gives a coloured product. Its structure would be
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 95) Which of the following statements is true?
A)
A polymer of (\[{{C}_{2}}{{H}_{5}}OH<KCl<N{{a}_{2}}S{{O}_{4}}<B{{a}_{3}}{{(P{{O}_{4}})}_{2}}<L{{i}_{3}}P{{O}_{4}}\] -glucose is readily digested by human being and not that of \[B{{a}_{3}}{{(P{{O}_{4}})}_{2}}<{{C}_{2}}{{H}_{5}}OH<L{{i}_{3}}P{{O}_{4}}<{{C}_{2}}{{H}_{5}}OH<KCl\] -glucose
done
clear
B)
A polymer of \[B{{a}_{3}}{{(P{{O}_{4}})}_{2}}<N{{a}_{2}}S{{O}_{4}}<L{{i}_{3}}P{{O}_{4}}<{{C}_{2}}{{H}_{5}}OH<KCl\] -glucose is readily digested by human beings and not that of \[(p{{H}_{1}})\] -glucose
done
clear
C)
Polymers of both a and (\[(p{{H}_{2}})\] -glucose are not readily digested by human beings
done
clear
D)
Polymers of both a and \[(p{{H}_{3}})\] -glucose are readily digested by human beings
done
clear
View Answer play_arrow
question_answer 96)
Predict the order of \[(p{{H}_{4}})\] for the following compounds. I. \[p{{H}_{1}}>p{{H}_{2}}>>p{{H}_{3}}>p{{H}_{4}}\] II. \[p{{H}_{1}}>p{{H}_{2}}>p{{H}_{3}}>p{{H}_{4}}\] III. \[p{{H}_{1}}<p{{H}_{2}}<p{{H}_{3}}>>p{{H}_{4}}\]
A)
\[p{{H}_{1}}<p{{H}_{2}}<p{{H}_{3}}<p{{H}_{4}}\]
done
clear
B)
\[{{P}_{T}}={{x}_{1}}p_{1}^{0}+{{x}_{2}}p_{2}^{0}\]
done
clear
C)
\[{{P}_{T}}=(1-{{x}_{2}})p_{1}^{0}+{{x}_{2}}p_{2}^{0}\]
done
clear
D)
\[{{P}_{T}}=p_{1}^{0}+(p_{2}^{0}-p_{1}^{0}){{x}_{2}}\]
done
clear
View Answer play_arrow
question_answer 97)
Arrange the following compounds in the order of increasing acidity \[\begin{align} & Benzyl\text{ }alcohol \\ & I \\ \end{align}\] \[\begin{align} & Benzoic\text{ }acid \\ & II \\ \end{align}\] \[\begin{align} & o\text{ }-\text{ }cresol \\ & III \\ \end{align}\] \[\begin{align} & Formic\text{ }acid \\ & IV \\ \end{align}\]
A)
\[({{E}^{o}})\]
done
clear
B)
\[OC{{l}^{-}}/C{{l}^{-}}\]
done
clear
C)
\[C{{l}^{-}}/\frac{1}{2}C{{l}_{2}}\]
done
clear
D)
\[1.36\text{ }V\]
done
clear
View Answer play_arrow
question_answer 98)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 99) \[{{E}^{o}}\]I, II and III are
A)
I II III \[C{{l}^{-}}/\frac{1}{2}C{{l}_{2}}\] heat \[-0.42V\] heat C, heat
done
clear
B)
I II III \[-0.36V\], C, heat C, heat
done
clear
C)
I II III \[0.58V\] \[1.17V\] \[CH_{3}^{+}\]
done
clear
D)
I II III \[NH_{2}^{-}\] C, heat C, heat
done
clear
View Answer play_arrow
question_answer 100) Bakelite is made from phenol and formaldehyde. The initial reaction between the two compounds is an example of
A)
free radical reaction
done
clear
B)
aldol reaction
done
clear
C)
aromatic electrophilic substitution
done
clear
D)
aromatic nucleophilic substitution
done
clear
View Answer play_arrow
question_answer 101)
Which of the following will be true if a cell is small in size? I. Nucleus will be small. II. Nucleus will be very large. III. It will be metabolically more active. IV. It will be metabolically less active.
A)
I and III
done
clear
B)
Only III
done
clear
C)
I and IV
done
clear
D)
II and III
done
clear
View Answer play_arrow
question_answer 102) Which one is correctly matched?
A)
Animal Characteristic Taxon Millipede Verbal nerve cord Verbal nerve cord Arachnids
done
clear
B)
Animal Characteristic Taxon Silver fish Pectoral and pelvic fins Chordata
done
clear
C)
Animal Characteristic Taxon Duck-billed Platypus Oviparous Mammalia
done
clear
D)
Animal Characteristic Taxon Sea anemone Triploblastic Cnidaria
done
clear
View Answer play_arrow
question_answer 103)
Read the following statement and select the correct option. I. Circulatory system in arthropods is of closed type. II. Parapodia in annelids help in swimming. III. Phylum-Mollusca is the second largest animal phylum. IV. Aschelminthes are dioecious.
A)
Only I
done
clear
B)
Only III
done
clear
C)
Only IV
done
clear
D)
III and IV
done
clear
View Answer play_arrow
question_answer 104)
Select the animal which has the following features is their body. I. Marine habitat. II. Bilateral symmetry and cephalisation. III. Haemocoel as principal body cavity. IV. Eyes similar to that of vertebrates.
A)
silver fish
done
clear
B)
dog fish
done
clear
C)
cattle fish
done
clear
D)
jelly fish
done
clear
View Answer play_arrow
question_answer 105) Which of the following is properly matched?
A)
Platy helminthes - Trematoda - Planaria
done
clear
B)
Echinodermata - Asteroidea - Star fish
done
clear
C)
Arthropoda - Insecta - Spider
done
clear
D)
Mollusca - Cephalopoda - Unio
done
clear
View Answer play_arrow
question_answer 106)
Match the following column I with column II. Column I Column II A. Cyclostone 1. Hemichordata B. Aves 2. Urochordata C. Tunicates 3. Agnatha D. Balanoglossus 4. Pisce E. Osteichthyes 5. Tetrapoda
Codes
A)
A-1 B-2 C-3 D-4 E-5
done
clear
B)
A-2 B-3 C-4 D-1 E-5
done
clear
C)
A-3 B-5 C-2 D-1 E-4
done
clear
D)
A-3 B-1 C-5 D-2 E-4
done
clear
View Answer play_arrow
question_answer 107)
Which of the following statement (s) is/are true in regard to human digestion? I. Liver, the largest gland of human body secretes, anticoagulant heparin. II. Pancreas, the endocrine gland secretes pancreatic juice which in slightly acidic in nature. III. Both carbohydrates and lipids are majorly absorbed into blood capillaries in small in intestine. IV. Trypsinogen is converted to trypsin with the help of enterokinase, present in intestinal, juice.
A)
Only I
done
clear
B)
I, II and III
done
clear
C)
III and IV
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 108)
Match the following column I with colunm II. Column I Column II A. Earthworm 1. Gizzard B. Cockroach 2. Caecum C. Frog 3. Clitellum D. Rat 4. Cloaca
Codes
A)
A-1 B-2 C-4 D-3
done
clear
B)
A-3 B-1 C-4 D-2
done
clear
C)
A-2 B-1 C-3 D-4
done
clear
D)
A-3 B-1 C-2 D-4
done
clear
View Answer play_arrow
question_answer 109) Which one of the following phyla is correctly matched with its two general characteristics?
A)
Echinodermata - Pentamerous radial symmetry and mostly internal fertilization
done
clear
B)
Mollusca- Normally oviparous and development through a trochophore or veliger larva
done
clear
C)
Arthropoda - Body is divided into head, thorax and abdomen and respiration by tracheae
done
clear
D)
Chordata - Notochord at some stage and separate anal and urinary opening to the outside
done
clear
View Answer play_arrow
question_answer 110) Respiratory, blood vascular and skeletal systems are absent in the members of phylum.
A)
Annelida
done
clear
B)
Porifera
done
clear
C)
Platyhelminthes
done
clear
D)
Echinodermata
done
clear
View Answer play_arrow
question_answer 111) Foramen trans versarium, also called vertebro-arterial canal is present in all
A)
cervical vertebrae
done
clear
B)
sacral vertebrae
done
clear
C)
lumber vertebrae
done
clear
D)
caudal vertebrae
done
clear
View Answer play_arrow
question_answer 112) Glissons capsule, the thin sheet of connective tissue cover the
A)
liver
done
clear
B)
kidney
done
clear
C)
stomach
done
clear
D)
pancreas
done
clear
View Answer play_arrow
question_answer 113)
SCID or Severe Combined Immuno Deficiency Syndrome can be used permanently by I. Enzyme replacement therapy. II. By SIRNA approach. III. Introducing bone marrow cells producing ADA into cells at early embryonic stages. IV. By inserting the functional gene using retroviruses. V. Periodic infusion of genetically engineered lymphocytes having functional ADA, c DNA.
A)
II, III and IV
done
clear
B)
III and IV
done
clear
C)
III and V
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 114) The grey crescent is the area
A)
at the point of entry of sperm into ovum
done
clear
B)
just opposite to the site of entry of sperm into ovum
done
clear
C)
at the animal pole
done
clear
D)
at the vegetal pole
done
clear
View Answer play_arrow
question_answer 115)
RBCs and adipocytes respire anaerobically because they I. require much less energy. II. possess carbonic anhydrase. III. possess very few mitochondria. IV. possess few mitochondria and large amount of energy.
Choose the correct option.
A)
I and II
done
clear
B)
I and III
done
clear
C)
II and IV
done
clear
D)
I, II and III
done
clear
View Answer play_arrow
question_answer 116)
Choose the correct statements. I. Ova collected from a female donor are transferred to the Fallopian tube to facilitate zygote formation in artificial insemination. II. Zygote is collected from a female donor and transferred to the Fallopian tube in IVF. III. Transfer of an ovum collected from a donor info the Fallopian tube of another female who cannot produce an ovum is called as GIFT. IV. Transfer of early embryos with up to 8-blastomeres into the Fallopian tube of the female is called ZIFT.
A)
I, II and IV
done
clear
B)
II, III and IV
done
clear
C)
Only II
done
clear
D)
III and IV
done
clear
View Answer play_arrow
question_answer 117) How is the arrangement of Mendels selected seven characters on four chromosomes?
A)
One is chromosome number 1, four in chromosome number 4, one in chromosome number 5 and one in chromosome number 7
done
clear
B)
Two in chromosome number 1, Three in chromosome number 4, one in chromosome number 5 and one in chromosome number 6
done
clear
C)
Three in chromosome number 1, one in chromosome number 4, two in chromosome number 5 and one in chromosome number 7
done
clear
D)
Two in chromosome number 1, Three in chromosome number 4, One in chromosome number 5 and one in chromosome number 7
done
clear
View Answer play_arrow
question_answer 118)
Choose the correct sequence for evolution of human ancertors. I. Dryopithecus II. Ramapithecus III. Homo erectus IV. Australopithecus
A)
\[I\to II\to III\to IV\]
done
clear
B)
\[IV\to III\to 1\to II\]
done
clear
C)
\[I\to II\to 1V\to III\]
done
clear
D)
\[II\to I\to 1V\to III\]
done
clear
View Answer play_arrow
question_answer 119) AABbCc genotype forms how many types of gametes?
A)
4
done
clear
B)
8
done
clear
C)
2
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 120)
Which statement (s) is/are correct? I. Degeneracy of code in related to third member of codon. II. Single codon codes for more than one amino acid. III. In codon, first two bases are more specific. IV. In codons, third base is wobble. V. Code is universal.
A)
I, II, III, IV and V
done
clear
B)
I, II and IV
done
clear
C)
I, III and IV
done
clear
D)
I, III. IV and V
done
clear
View Answer play_arrow
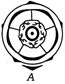
question_answer 121)
An impairement affecting synthesis or release of ADH result in a diminished ability of the kidney to conserve water leading to water loss and dehydration. This hormone released in blood by which of the following labelled structure in the give diagram?
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D and A
done
clear
View Answer play_arrow
question_answer 122)
Select the correct matches. Column I Column II I. Euglena Animal and plant II. Archaeopteryx Reptiles and Mammalia III. Balanoglossus Eurochordata and Cephatochordata IV. Penpatus Annelida and Arthropoda
A)
I and IV
done
clear
B)
II and IVV
done
clear
C)
I and III
done
clear
D)
II and IV
done
clear
View Answer play_arrow
question_answer 123) Bombyx mori a silk math is a
A)
holomata bola - insect-like mosquito
done
clear
B)
hemimata bola - insect-like bed bug
done
clear
C)
holomata bola - insect like-cockroach
done
clear
D)
hemimata bola - insect-like butterfly
done
clear
View Answer play_arrow
question_answer 124) Erythroblastosis foetalis is caused if the fertilisation occurs between gametes of
A)
\[R{{h}^{-}}\] female and \[R{{h}^{+}}\] male
done
clear
B)
\[R{{h}^{+}}\] female and \[R{{h}^{-}}\] male
done
clear
C)
\[R{{h}^{-}}\]female and \[R{{h}^{+}}\]male
done
clear
D)
\[R{{h}^{+}}\] female and \[R{{h}^{-}}\] male
done
clear
View Answer play_arrow
question_answer 125)
Arrange the following in order of accurancy needed to perform the process given below. I. Replication II. Transcription III. Translation
A)
I > II > III
done
clear
B)
III > II > I
done
clear
C)
I > III > II
done
clear
D)
II > I > III
done
clear
View Answer play_arrow
question_answer 126) A, B and C are the three forms of DNA. In which
A)
A and B are right handed and C is left handed
done
clear
B)
A is right handed and B and C are left handed
done
clear
C)
All three are right handed, but the number of bases per turn in the three is different
done
clear
D)
AII three are left hand, but the number of bases per turn in the three is different
done
clear
View Answer play_arrow
question_answer 127)
Mark the incorrect statement (s). I. Protoplasm is a physical basis of the life is said by Rudoef Virchow. II. Haeckal is well known for his recapitulation theory. III. TH Morgan gave modern principle of inheritance. IV. Chromosomal theory of inheritance is given by Me Leod and Me Carty.
A)
I and IV
done
clear
B)
II and III
done
clear
C)
I and III
done
clear
D)
II and IV
done
clear
View Answer play_arrow
question_answer 128) Following are given some statements regarding nodes of Ranvier. Which of the following in correct for nodes of Ranvier of nerve?
A)
Neurilemma is discontinuous
done
clear
B)
Myelin sheath is discontinuous
done
clear
C)
Both neurilemma and myelin sheath are discontinuous
done
clear
D)
Covered by myelin sheath
done
clear
View Answer play_arrow
question_answer 129)
Which of the following statements is/are wrong regarding ZIFT? I. It stands for Zygote Intra Fallopian Transfer. II. Zygote is transferred into the Fallopian tube after IVF. III. Early embryos up to 8-blastomeres can also be transferred into the Fallopian tubes. IV. Embryos with more than 8-blastomeres are also transferred into the Fallopian tubes.
A)
I, II and III
done
clear
B)
Only I
done
clear
C)
II and IV
done
clear
D)
Only IV
done
clear
View Answer play_arrow
question_answer 130) A cardiac cycle includes
A)
auricular systole-ventricular diastole-joint diastole
done
clear
B)
auricular systole-ventricular systole-complete cardiac diastole
done
clear
C)
ventricular diastole - auricular systole - complete cardiac diastole
done
clear
D)
joint diastole-ventricular systole-complete cardiac diastole
done
clear
View Answer play_arrow
question_answer 131) Given that I = natural selection; II = variation and their inheritance; III = survival of the fittest; IV = struggle for existence. According to Darwinism, which of the following represents the correct sequence of events in the origin of new species?
A)
III, IV, I and II
done
clear
B)
II,III, I and IV
done
clear
C)
I, II, III and IV
done
clear
D)
IV, II, III and I
done
clear
View Answer play_arrow
question_answer 132)
EEG is an idex of brain functioning and is most useful in the diagnosis of I. epilepsy. II. brain infections and drug effects on the brain. III. sleep and it disorder. IV. brain death.
A)
I and II
done
clear
B)
I and III
done
clear
C)
I, II and III
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 133)
Match the following column I with column ll. Column I Column ll A. Accessary duct 1. Seminal vesicle B. Accessary gland 2. Vas defference C. Spermatids 3. Uterus D. Primary spermatocyte 4.23-chromosomes 5. 46 -chromosomes
Codes
A)
A-4,3 B-5 C-2 D-1
done
clear
B)
A-4,5 B-2 C-1 D-3
done
clear
C)
A-2,1 B-3 C-5 D-4
done
clear
D)
A-2,3 B-1 C-4 D-5
done
clear
View Answer play_arrow
question_answer 134)
Consider the following statements and mark them as true or false. I. Ligaments are connective tissues that connect skeletal muscles to bones. II. Bone marrow is composed of adipose tissue, areolar tissue and blood. III. Hyaline cartilage in the strogest cartilage in vertebrate body. IV. Mast cells are modified basophils which secrete vaso constrictor, i.e. histamine.
True false
A)
II, III I, IV
done
clear
B)
ll, IV I. Ill
done
clear
C)
I, IV II, III
done
clear
D)
III,IV I, II
done
clear
View Answer play_arrow
question_answer 135) In Drosophila the XXY condition leads to femaleness whereas in human beings the same condition leads to Klinefelterss syndrome in male. It proves
A)
in human being Y-chromosome is active in sex determination
done
clear
B)
Y-chromosome is active in sex determination in both human beings and Drosophila
done
clear
C)
in Drosophila Y-chromosome decides femaleness
done
clear
D)
Y-chromosome of man have genes for syndrome
done
clear
View Answer play_arrow
question_answer 136) Salivary glands are absent in
A)
Anopheles maculipennis
done
clear
B)
Musca domestica
done
clear
C)
Blatta orientalis
done
clear
D)
Rana hexadactyla
done
clear
View Answer play_arrow
question_answer 137)
Which of following some as biological control of plant pests? I. Lady beetles II. Leopard moth III. Stink bugs IV. White grubs
A)
Only I
done
clear
B)
I and III
done
clear
C)
II and IV
done
clear
D)
III and IV
done
clear
View Answer play_arrow
question_answer 138)
Match the following column I with column II. Column I Column II A. UUU 1. Serine B. GGG 2. Methionine C. UCU 3. Phenyl alanine D. AUG 4. Glycine E. CCC 5. Proline
Codes
A)
A-3 B-4 C-1 D-2 E-5
done
clear
B)
A-1 B-2 C-3 D-4 E-5
done
clear
C)
A-3 B-4 C-5 D-1 E-2
done
clear
D)
A-4 B-3 C-1 D-2 E-3
done
clear
View Answer play_arrow
question_answer 139) Corpus luteum remains active during early pregnancy producing progesterone because of human Chorionic Gonadotropin (hCG). But in the later stages the production of progesterone is taken over by
A)
placenta
done
clear
B)
allantois
done
clear
C)
chorion
done
clear
D)
amnion
done
clear
View Answer play_arrow
question_answer 140) In human gut in order of secretion, the proteolytic enzymes are
A)
renin, trypsin and steapsin
done
clear
B)
pepsin, trypsin and erepsin
done
clear
C)
trypsin, steapsin and dipeptidase
done
clear
D)
None of the the above
done
clear
View Answer play_arrow
question_answer 141) Which of the following nervous system is called cranial-sacral out flow?
A)
SNS and it is adrenergic
done
clear
B)
PSNS and it is adrenergic
done
clear
C)
SNS and it is cholinergic
done
clear
D)
PSNS and it is cholinergic
done
clear
View Answer play_arrow
question_answer 142) The endogenous pyrogen (mterleukin-I) is secreted by
A)
neutrophils
done
clear
B)
eosinophils
done
clear
C)
macrophages
done
clear
D)
monocytes
done
clear
View Answer play_arrow
question_answer 143)
During the study of sexually transmitted disease, a student learns about there mode of transmission among individuals, their casuative agents, etc. Correctly match the agents and the STDs they cause given in column I and II. Column I Column II A. Treponema pallidum 1. Chlamydia B. HSV type-l 2. Syphillis C. Trichomonas vaginalis 3. Genital warts D. Chlamydia trachomatis 4. Trichomoniasis
Codes
A)
A-1 B-2 C-3 D-4
done
clear
B)
A-2 B-3 C-4 D-1
done
clear
C)
A-4 B-3 C-2 D-1
done
clear
D)
A-3 B-4 C-1 D-2
done
clear
View Answer play_arrow
question_answer 144)
Match the following column I with column II. Column I Column II A. Struthio 1. Retrogressive metamorphis B. Ichthyophis 2. Largest living flightless bird C. Herdmania 3. Flying bird D. Psittacula 4. A limbless amphibian
Codes
A)
A-1 B-2 C-3 D-4
done
clear
B)
A-2 B-4 C-1 D-3
done
clear
C)
A-4 B-3 C-2 D-1
done
clear
D)
A-3 B-2 C-4 D-1
done
clear
View Answer play_arrow
question_answer 145) Gravi-index test (pregnancy test) detects the presence of following hormone in urine.
A)
hCG
done
clear
B)
Progesterone
done
clear
C)
Relaxin
done
clear
D)
FSH
done
clear
View Answer play_arrow
question_answer 146) Trophoblast of the blast cyst gets differentiated into
A)
allantois
done
clear
B)
chorion
done
clear
C)
amnion
done
clear
D)
embryo
done
clear
View Answer play_arrow
question_answer 147) Identify the correctly matched pair.
A)
Acrodont - Teeth attached to bony sockets
done
clear
B)
Thecodont - Teeth attached to the rest of the bone
done
clear
C)
Pleurodont - Teeth attached to the medial side of bone
done
clear
D)
Diphyodont - Teeth appearing many times in life
done
clear
View Answer play_arrow
question_answer 148)
Which of the following statements is/are true? I. Urine is hypotonic in distal convoluted tubule. II. When the urine passes into the collecting tubule it becomes hypotonic. III. Urine is isotonic in proximal convoluted tubule. IV. Urine becomes more and more hypotonic as it passes through the loop of Henle.
A)
I and IV
done
clear
B)
I, II and III
done
clear
C)
II and HI
done
clear
D)
Only III
done
clear
View Answer play_arrow
question_answer 149)
Match the following column I with column II. Column I Column II A. Bee wax 1, Five year life span B. Pebrine 2. Worker bees C. Queen bee 3. Disease of silkworms D, Bombax mori 4. Insect E. Worker bee 5. 90 days of life span
Codes
A)
A-2 B-3 C-1 D-4 E-5
done
clear
B)
A-5 B-4 C-3 D-2 E-1
done
clear
C)
A-3 B-4 C-5 D-1 E-2
done
clear
D)
A-1 B-3 C-4 D-5 E-2
done
clear
View Answer play_arrow
question_answer 150) Opisthonephron kidney is the modification of
A)
mesonephrons kidney
done
clear
B)
pronephrons kidney
done
clear
C)
metanephrons kidney
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 151) A major breakthrough in the studies of cells come with the development of electron microscope. This is because
A)
the electron microscope is more powerful than the light microscope as it uses a beam of electrons which has wavelength much longer than that of photons
done
clear
B)
the resolving power of electron microscope is much higher than that of the light microscope
done
clear
C)
the resolving power of the electron microscope is 200-350 nm as compared to 0.1-0.2 nm for the light microscope
done
clear
D)
electron beam can pass through thick materials whereas light microscope requires their section
done
clear
View Answer play_arrow
question_answer 152)
Match the following column I with column II. Column I (Author) Column II (Book/Monograph) A. Andreas Vesalius 1. Micrographia B. Carolus Linneus 2. Philosophic zoologique C. Robert Hook 3. De Humani corpons Fabrica D. Jean Baptiste de 4. Species Plantarum Lamarck
Codes
A)
A-4 B-2 C-3 D-1
done
clear
B)
A-3 B-4 C-1 D-2
done
clear
C)
A-4 B-3 C-2 D-1
done
clear
D)
A-3 B-2 C-4 D-1
done
clear
View Answer play_arrow
question_answer 153)
Choose the correct statement. I. The rich sources of iodine are Laminaria Fucus and Aascophyllum. II. Volvox globalor and V. aureus are colonial flagellates. III. There is no flagellated phage in life cycle of Spirogyra. IV. Chlamydomonas, does not reproduce sexually.
A)
Only I
done
clear
B)
Only II
done
clear
C)
Only III
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 154)
Identify the suitable matching pairs from column I and column II. Column I Column II A. Peritrichous flagella 1. Macrocystis B. Rhizophore 2. Ginkgo C. Largest perennial alga 3. E. coli D. Living fossil 4. Wolffia E. Smallest flowering plant 5. Selagineila
Codes
A)
A-4 B-3 C-5 D-2 E-1
done
clear
B)
A-3 B-5 C-4 D-1 E-2
done
clear
C)
A-1 B-3 C-5 D-2 E-4
done
clear
D)
A-3 B-5 C-1 D-2 E-4
done
clear
View Answer play_arrow
question_answer 155) The family containing mustard and its main characters are
A)
Brassicaceae - Tetramerous flowers, six stamens, bicarpellary gynoecium, capsuletype fruit
done
clear
B)
Brassicaceae - Pentamerous flowers, many stamens pentacarpellary gynoecium, capsule type fruit
done
clear
C)
Solanaceae-Pentamerous flower, five stamens, bicarpellary gynoecium berry type fruit
done
clear
D)
Poaceae- flowers, three stamens, monocarpellary gynoecium, caryopsis type of fruit
done
clear
View Answer play_arrow
question_answer 156)
Match the term in column I with their description in column II. Column I Column II A. Fossil 1. Accumulation of neutral mutations B. Stratification 2. is shark fins, pengen in flippers C. Molecular clock 3. Stone hand evidence of life in past D. Analogy 4. Lyer of sedimentary rock
Codes
A)
A-4 B-2 C-3 D-1
done
clear
B)
A-3 B-4 C-1 D-2
done
clear
C)
A-4 B-1 C-2 D-1
done
clear
D)
A-3 B-2 C-4 D-2
done
clear
View Answer play_arrow
question_answer 157) Choose the correctly matched pair
A)
Honey mushroom -ArmiHaria
done
clear
B)
Birds nest fungus - Phallu
done
clear
C)
Tooth fungi - Hydnum
done
clear
D)
Coral fungi - Clavaria
done
clear
View Answer play_arrow
question_answer 158)
Read the following statements. I. The four nucleotide basis are not necessarily present in DNA in exact equal proportions. II. The total amount of purines is equal to the total amount of pyrimidines. III. DNA ligase enzyme act to hydrolyse or breakdown a polynucleotide chain into its component nucleotide. IV. Nuclease enzyme are capable of restoring an intact DNA duplex of the above statements.
A)
I and II are incorrect, but III and IV are correct
done
clear
B)
I and II are correct, but IV and III are incorrect
done
clear
C)
II is correct, but I III and IV are incorrect
done
clear
D)
II, III and IV are correct but I is incorrect
done
clear
View Answer play_arrow
question_answer 159) What are the successive structures formed in case of sexual reproduction is Rhizopus?
A)
Zygospore, progametangium, gametangium, zygophore
done
clear
B)
Progametangium, zygophore, gametangium. Zygospore
done
clear
C)
Progametangium, gametangium, zygospore zygophore
done
clear
D)
Zygophore, progametangium, gametanQium, zygospore
done
clear
View Answer play_arrow
question_answer 160)
Match the following column I with column II. Column I Column II A. Chargaff 1. Wilkins and Franklin B. Replicon 2. Uptake of lactose C. Permease 3. hnRNA D. Split gene 4. Length of DNA E. X-ray diffraction 5. \[(A+G)=(C+T)\]
Codes
A)
A-2 B-3 C-4 D-1 E-5
done
clear
B)
A-5 B-4 C-2 D-3 E-1
done
clear
C)
A-1 B-3 C-2 D-4 E-5
done
clear
D)
A-2 B-4 C-1 D-3 E-5
done
clear
View Answer play_arrow
A)
A-3 B-4 C-1 D-2
done
clear
B)
A-2 B-1 C-4 D-3
done
clear
C)
A-1 B-3 C-4 D-2
done
clear
D)
A-4 B-3 C-2 D-1
done
clear
View Answer play_arrow
question_answer 162)
Consider the following statements and choose the correct answers from the given codes I. The sporophyte in liverworts is more elaborate than that is noxes. II. Salvinia is heterosporous. III. The life cyclie in all seed bearing plant is diplontic. IV. In Pinus male and female cones are borne on different trees.
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 163)
Select the correct statement (s). I. Multicarpellary and syncarpous gynoecium is present in Michelia. II. Multicarpellary and syncarpous gynoecium is present in Papaver. III. Unicarpellary and apocarpous gynoecium is present in Papaver. IV. Multicarpellary and apocarpous gynoecium is present in Muchelia.
A)
I and II
done
clear
B)
I and III
done
clear
C)
II and IV
done
clear
D)
III and IV
done
clear
View Answer play_arrow
question_answer 164) Cell division was observed in the single cell of root tip. How many generation of mitosis are required to produce 128 cells from the single cell of the root tip?
A)
64
done
clear
B)
8
done
clear
C)
7
done
clear
D)
32
done
clear
View Answer play_arrow
question_answer 165)
Match the following column I with column II. Column I Column II A. Bacillus thuringiensis 1. Production of chitinases B. Rhizobium meliloti 2. Scavenging of oil spills C. Escherichiacoli 3. Incorporation of nif gene D. Pseudomonas putida 4. Production of Bt toxin E. Trichoderma 5. Production of human insulin
Codes
A)
A-2 B-3 C-4 D-1 E-5
done
clear
B)
A-4 B-2 C-5 D-3 E-1
done
clear
C)
A-4 B-3 C-5 D-2 E-1
done
clear
D)
A-5 B-4 C-3 D-2 E-1
done
clear
View Answer play_arrow
question_answer 166) All of the following statements concerning the Actinomycetes filamentous soil bacterium Fmnkia are correct except that Frankia.
A)
Can induce root nodules of many plant species
done
clear
B)
Can fix nitrogen in the free living state
done
clear
C)
Cannot fix specialised vesicles in which the nitogenase is protected from oxygen by a chemical barrier involving triterpene hapanoids
done
clear
D)
Like Rhizobium it usually infects its hort plant through root air deformation and stimulates cell proliferation in the hort cortex
done
clear
View Answer play_arrow
question_answer 167)
Consider the following given statements. Two statements are correct. Choose the correct options from the given codes. I. A lion eating a deer and a sparrow feeding an grain are ecologically similar in being consumers. II. Predator star fish Pisaster helps in maintaining species diversity of some invertebratess. III. Predators ultimately lead to the extinction of prey species. IV. Production of chemicals such as nicotine, strychnine by the plants are metabolic disorders.
A)
Hand III
done
clear
B)
III and IV
done
clear
C)
I and IV
done
clear
D)
I and II
done
clear
View Answer play_arrow
question_answer 168)
Consider the following statements and select true or false. I. A constant input of solar energy is basic requirement of ecosystem. II. Biomass or organic matter produced is expressed in terms of K cal \[{{m}^{-2}}\]. III. The rate of biomass production is called net primary production. IV. Net primary productivity minus respiration called gross primary production.
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
A)
A B C Liliaceae Asteraceae Solanaceae
done
clear
B)
A B C Asteraceae Solanaceae Brassicaceae
done
clear
C)
A B C Asteraceae Fabaceae Poaceae
done
clear
D)
A B C Poaceae Solanaceae Asteraceae
done
clear
View Answer play_arrow
question_answer 170)
Match the following Column I with Column II. Column I Column II A. Pacific salmon fish 1. Verhulst-pearl logistic growth B. \[{{N}_{1}}={{N}_{0}}{{e}^{rt}}\] 2. Breeds only once in lifetime C. Oyster 3. Exponential growth D. \[\frac{dN}{dt}=rN\left( \frac{K-N}{K} \right)\] 4- A large number of small sized offsprings
A)
A-4 B-3 C-1 D-2
done
clear
B)
A-3 B-4 C-1 D-2
done
clear
C)
A-3 B-1 C-4 D-2
done
clear
D)
A-2 B-3 C-4 D-1
done
clear
View Answer play_arrow
question_answer 171) The orderly change in A leads some change in community that is near B with the environment. This community is known as C community and the process is termed Fill in the blanks with suitable words.
A)
A B C D Energy Optimum Serai Development
done
clear
B)
A B C D Radiation Minimum Pioneer Coevolutionv
done
clear
C)
A B C D Environment Equilibrium Climax Ecological succession
done
clear
D)
A B C D Wind flow Maximum Decomposer Secondary succession
done
clear
View Answer play_arrow
question_answer 172)
Match the following column I with column II. Column I Column II A. Aerobic 1. Frankia B. Cyanobacteria 2. Azospirillum C. Casuarina 3. Clostridium D. Tropical grasses 4. Aulozira 5. Azotobacter
Codes
A)
A-3 B-5 C-4 D-2
done
clear
B)
A-2 B-1 C-3 D-5
done
clear
C)
A-5 B-3 C-4 D-1
done
clear
D)
A-5 B-4 C-1 D-2
done
clear
View Answer play_arrow
question_answer 173) If a recombinant DNA bearing gene for amplicillin resistance is transferred into E. coli cells and the host cells are spread an agar plates containing amplicillin, then
A)
both transformed and untransformed recipient cells will die
done
clear
B)
both transformed and untransformed recipient cells will grow
done
clear
C)
transformed recipient cells will grew and untransformed recipient cells will die
done
clear
D)
transformed recipient cells wilt die and untransformed recipient cells will grew
done
clear
View Answer play_arrow
question_answer 174)
Match the following column I with column II. Column I Column II A. Red algae 1. Marchantia B. Liverwort 2. Pinus C. Walking fern 3. Polysiphonia D. Gymnosperm 4. Adiantum
Codes
A)
A-3 B-5 C-4 D-3
done
clear
B)
A-2 B-4 C-3 D-1
done
clear
C)
A-2 B-3 C-1 D-4
done
clear
D)
A-3 B-1 C-4 D-2
done
clear
View Answer play_arrow
question_answer 175)
Match the following column I with column II. Column I Column II A. Bacillus thringiensis 1. Production of human insulin, growth hormone B. Rhizobium meliloti 2. Production of Bt toxin C. Pseudomonas putida 3. Scanenging of oil spills D. Escherichia coli 4. \[{{N}_{2}}\] -fixation by n if gene in cereal crops
Codes
A)
A-2 B-4 C-3 D-1
done
clear
B)
A-1 B-2 C-3 D-4
done
clear
C)
A-2 B-1 C-4 D-3
done
clear
D)
A-2 B-3 C-1 D-2
done
clear
View Answer play_arrow
question_answer 176)
Match the following column I with column II. Column I Column II A. Peritrichous flagellation 1. Ginko B. Living fomils 2. Macrocystis C. Rhizophore 3. Escherichia coli D. Smallest flowering plant D. Smallest flowering plant E. Largest perevialagae 5. Woiffia
Codes
A)
A-3 B-1 C-4 D-5 E-2
done
clear
B)
A-2 B-1 C-3 D-4 E-5
done
clear
C)
A-4 B-3 C-2 D-5 E-1
done
clear
D)
A-1 B-2 C-5 D-3 E-4
done
clear
View Answer play_arrow
question_answer 177)
Read the following statements. I. Colchicin inhibits the addition oftubutin molecules to microfilaments, thus is a mitotic poison. II. The cell wall consists of cellulose, hemi cellulose, pectin, lignin and lipids. III. The urea cycle is compartmentacised between the matrix of the mitochondria and the cyto IV. Anthocyanin is a glucoside pigment, violet is neutral, red in acid and blue in alkaline cell sap.
Choose the correct option.
A)
I and II
done
clear
B)
I, II and III
done
clear
C)
I, II and IV
done
clear
D)
I, III and IV
done
clear
View Answer play_arrow
question_answer 178) \[2N{{H}_{3}}+3{{O}_{2}}\xrightarrow{A}2NO_{2}^{-}+2{{H}^{+}}+2{{H}_{2}}O\]\[2NO_{2}^{-}+{{O}_{2}}\xrightarrow{B}2NO_{3}^{-}\]Identify the organism and [B] involved in the above reactions respectively.
A)
Thiobacillus, Pseudomonas
done
clear
B)
NitrosomGnas, Nitrococcus
done
clear
C)
Nitrococcus, Nitrobacter
done
clear
D)
Azotobacter, Nitrococcus
done
clear
View Answer play_arrow
question_answer 179) Choose the incorrect option amongst the following.
A)
Photosynthetic pigment Name of organism Absorption(nm) Chlorophyll-d Red algae 740 nm
done
clear
B)
Photosynthetic pigment Name of organism Absorption(nm) p-caroten Green plants 450-480 nm
done
clear
C)
Photosynthetic pigment Name of organism Absorption(nm) Phycocyanin BGA 618nm
done
clear
D)
Photosynthetic pigment Name of organism Absorption(nm) Bacterioviridin All photosynth- eticbacteria 400-700 nm
done
clear
View Answer play_arrow
question_answer 180)
Read the following statements about frog and choose the correct option. I. The body colour offers it protective colouration. II. Sammer sleep of frog is called aestivation. III. Tail is not present in life cycle of frog. IV. Mouth is bounded by a pair of lips.
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 181) The genetic drift operates only in
A)
island population
done
clear
B)
smaller population
done
clear
C)
larger population
done
clear
D)
Mendelian population
done
clear
View Answer play_arrow
question_answer 182)
Identify which of the following utilities are offered to the service of man-kind by conservation of biodiversity? I. Ecosystem service like photosynthesis II. Industrial products like dyes and lubricants. III. Watching spring flowers in full bloom. IV. The aesthetic pleasure of walking through thick. V. Fibre, firewood and construction material. VI. Products of medicinal importance.
Choose the correct options.
A)
I, II and III
done
clear
B)
l,V and VI
done
clear
C)
IV, V and VI
done
clear
D)
I, III and VI
done
clear
View Answer play_arrow
question_answer 183)
Which of the following statement(s) is/are correct? I. Enzymes for peroxide biosynthesis lies within peroxisomes. II. Catalase enzyme is present in glyoxysomes, a cell organelle present in animal and plant cells. III. Centrioles play a role in aster formation during cell division in plants. IV. Ribozymes are proteins that play an important role during protein synthesis.
A)
Only I
done
clear
B)
I, II and III
done
clear
C)
I and IV
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 184) C-value means
A)
hapioid DNA content in an individual
done
clear
B)
colchiceni treatment value
done
clear
C)
gene binding frequency on chromosomes
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 185) Parbhani kranti is a variety of Abelomoschus esculentus, resistant to yellow mosaic virus was developed by
A)
plant breeding
done
clear
B)
plant tissue culture
done
clear
C)
mutational breeding
done
clear
D)
r DNA technology
done
clear
View Answer play_arrow
question_answer 186) Bryophyte used as antiseptic absorbant bandage is
A)
Sphagnum
done
clear
B)
Ricda
done
clear
C)
Marchantia
done
clear
D)
Porella
done
clear
View Answer play_arrow
question_answer 187) An ambiguous codon in nature is
A)
UAG
done
clear
B)
UGA
done
clear
C)
UAA
done
clear
D)
UUU
done
clear
View Answer play_arrow
question_answer 188)
Match the following column I with column II. Column I (Population interaction) Column II (Examples) A. Mutualism 1. Ticks on dogs B. Commensalism 2. Baianus and chathamalus C. Parasitism 3. Sparrow and any Seeds D. Campetition 4. Epiphyte on a mango branch E. Predation 5. Orchid ophrys and
Codes
A)
A-1 B-5 C-4 D-3 E-2
done
clear
B)
A-2 B-1 C-5 D-4 E-3
done
clear
C)
A-3 B-2 C-1 D-5 E-4
done
clear
D)
A-5 B-4 C-1 D-2 E-3
done
clear
View Answer play_arrow
question_answer 189)
Which of the following environmental condition are essential for optimum growth of Mucoron a piece of bread? I. Temperature of about \[{{25}^{o}}C\]. II. Temperature of about \[{{5}^{o}}C\] III. Relative humidity of about \[5%\] IV. Relative humidity of about \[95%\] V. A shady place. VI. A brightly illuminated place.
Choose the correct options.
A)
II, III and VI
done
clear
B)
I. III and V
done
clear
C)
I. IV and V
done
clear
D)
II, IV and V
done
clear
View Answer play_arrow
question_answer 190)
Consider the following statements, choose correct options. I. Plant cells have centrioles which are almost absent in all animal cells. II. Ribosomes are the site of protein synthesis. III. The middle lamella is the layer mainly of calcium carbonate which holds the different neighbouring cells together. IV. In animal cells steroidal hormones are synthesised by smooth endoplasmic reticulum.
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 191) The two microbes found to be very useful in genetic engineering are
A)
Grown gall bacterium and Caenorhabditis
done
clear
B)
E. call and A. tumefaciens
done
clear
C)
V. cholerae and bacteriophage
done
clear
D)
Pseudomonas and Xanthomonas
done
clear
View Answer play_arrow
question_answer 192)
From which of the following algae, the agar-agar is commercially extracted? I. Gracillaria II. Fucus III. Sargassum IV. Gelidium V. Turbmalia
A)
III and V
done
clear
B)
II and III
done
clear
C)
IV and V
done
clear
D)
I and IV
done
clear
View Answer play_arrow
question_answer 193)
Column I lists the shape of chloroplast in green algae and column II shows the name of that algae. Column I Column II A. Cup-shaped 1. Ulothrix B. Girdle-shaped 2. Oedogonium C. Stellate 3. Chlamydomonas D. Reticulate 4. Zygonema
Codes
A)
A-2 B-4 C-3 D-1
done
clear
B)
A-3 B-1 C-4 D-2
done
clear
C)
A-3 B-4 C-2 D-1
done
clear
D)
A-4 B-3 C-1 D-2
done
clear
View Answer play_arrow
question_answer 194)
Choose the incorrect pairs from the following. I. Holotype-A specimen on which the original description of the species is based. II. Syntype-A specimen other than the holotype available with the author. III. Paratype-any of two or more specimens cited by author when there is no holotype. IV. Neotype-A specimen is designated when the original types are known to have been destroyed. V. Isotype-A duplicate of the type.
A)
I and II
done
clear
B)
II and III
done
clear
C)
IV and V
done
clear
D)
I and V
done
clear
View Answer play_arrow
question_answer 195)
Read the following statement. Choose the correct statement from options given in codes. I. When a fruit develops from inflorescence, it is comporite. II. Mesocarp is the edible part in apple. III. Gynobasic style is seen in ocimum. IV. Hypanthodium is a special type of inflorescence found in euphorbia species.
A)
I and IV
done
clear
B)
I and III
done
clear
C)
I and II
done
clear
D)
I, II and IV
done
clear
View Answer play_arrow
question_answer 196)
Consider the following statements. There statements describes the major pigment and stored food in different group of algae. Choose the correct options. I. In chlorophyceae the stored food material is starch and major pigment is chlorophyceae -a and d. II. In phaeophyceae, laminarin is the stored food material and chlorophyll a and b are major pigments. III. In rhodophyceae, floridean starch is the stored food and the major pigments are chlorophyll-a, d and phycoerythrin.
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 197)
Based on which features an underground stem be differentiated from root. I. Presence of nodes and internodes. II. Presence of axillary and terminal buds. III. Presence of cap and hairs. IV. Absence of scale leaves and adventitious roots arising from nodes. V. Presence of exogenous branches.
Choose the correct option.
A)
I. II and IV
done
clear
B)
I, II and V
done
clear
C)
II. Ill and V
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 198)
Match the following column I with column II. Column I Column II A. Vitamin-B rich SCP 1. Dunaliella salina B. Foxfire 2. Amanita muscaria C. Methionine deficient SCP 3. Candida lipolytica D. Lysin rich SCP 4. Armillana melka 5. Methylophilus methylotropus
Codes
A)
A-3 B-4 C-1 E-5
done
clear
B)
A-3 B-2 C-1 E-4
done
clear
C)
A-3 B-5 C-1 E-4
done
clear
D)
A-1 B-4 C-2 E-3
done
clear
View Answer play_arrow
question_answer 199) The given below are the organisms with their some specific role. Choose the incorrect pair.
A)
Biogas - Methanobacterium
done
clear
B)
Mycorrhiza - Glomus
done
clear
C)
Biocontrol agent - Pythium
done
clear
D)
Ladybird beetle - Aphid
done
clear
View Answer play_arrow
question_answer 200) Bentham and Hooker classified dicots into
A)
Polypetalae, gamopetalae and glumifloral
done
clear
B)
Polypetalae, gamopetalae monochlamydae
done
clear
C)
Archechlamydae, monochlamydae and glumifloral
done
clear
D)
Polypetalae, gamopetalae and monochlamydae
done
clear
View Answer play_arrow
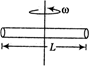
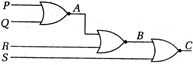
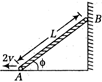
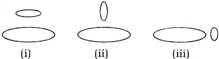
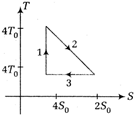
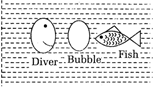
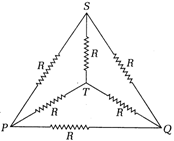
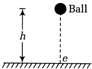
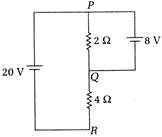
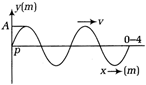
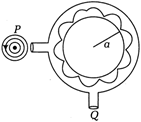
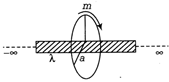
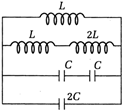
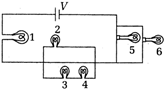
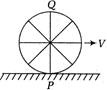
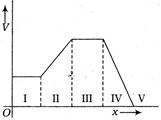
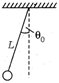
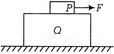
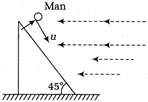 The speed of rain is
The speed of rain is
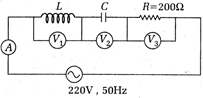
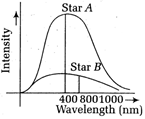
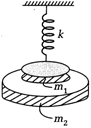
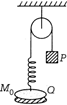
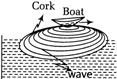
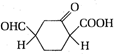
 Hydrogenation of the above compound in the presence of poisoned palladium catalyst gives
Hydrogenation of the above compound in the presence of poisoned palladium catalyst gives