A) zero
B) one
C) two
D) three
E) half
Correct Answer: A
Solution :
\[{{P}_{1}}=80\,kPa,({{t}_{1/2}})=350s\] \[{{P}_{2}}=40\,kPa,{{({{t}_{1/2}})}_{2}}=175s\] \[\frac{80}{40}=\frac{350}{175}=2\]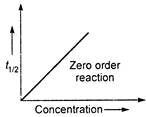 \[\because \] \[\frac{{{P}_{1}}}{{{P}_{2}}}=\frac{{{({{t}_{1/2}})}_{1}}}{{{({{t}_{1/2}})}_{2}}}=\frac{{{a}_{1}}}{{{a}_{2}}}\] \[\therefore \] \[={{t}_{1/2}}\propto a\](zero order reaction) Note: For\[{{n}^{th}}\]order reaction \[{{t}_{1/2}}\propto \frac{1}{{{(a)}^{n-1}}}\]
\[\because \] \[\frac{{{P}_{1}}}{{{P}_{2}}}=\frac{{{({{t}_{1/2}})}_{1}}}{{{({{t}_{1/2}})}_{2}}}=\frac{{{a}_{1}}}{{{a}_{2}}}\] \[\therefore \] \[={{t}_{1/2}}\propto a\](zero order reaction) Note: For\[{{n}^{th}}\]order reaction \[{{t}_{1/2}}\propto \frac{1}{{{(a)}^{n-1}}}\]
You need to login to perform this action.
You will be redirected in
3 sec
