A) 1, 3-dichloropropane
B) 1, 2-dichloropropane
C) 2, 2-dichloropropane
D) 1, 1-dichloropropane
E) 1, 3-dichloropropene
Correct Answer: D
Solution :
\[X\]is a three carbon compound with two halogen atom, so its molecular formula is\[{{C}_{3}}{{H}_{6}}C{{l}_{2}}\]Only terminal alkynes give red ppt with ammoniacal\[CuC{{l}_{2}},\]so the hydrocarbon produced by the reaction of X with ale KOH, must be a terminal alkyne (ie,\[C{{H}_{3}}C\equiv CH\]) \[{{C}_{3}}{{H}_{6}}C{{l}_{2}}\xrightarrow[{}]{Alc\,KOH}C{{H}_{3}}C\equiv CH\xrightarrow[{}]{Amm\,C{{u}_{2}}C{{l}_{2}}}\] \[\underset{\operatorname{Re}d\,ppt}{\mathop{C{{H}_{3}}C\equiv CCu}}\,\downarrow \] Compound (X) gives an aldehyde when reacts with aqueous KOH. This suggests that both the halogens are present on same terminal carbon atom. Thus, the formula of compound (X) is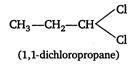 and the reactions are as follows:
and the reactions are as follows: 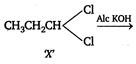 \[C{{H}_{3}}C\equiv CH\xrightarrow[C{{u}_{2}}C{{l}_{2}}]{Ammoniacal}\underset{\operatorname{Re}d\,ppt}{\mathop{C{{H}_{3}}C\equiv CCu}}\,\downarrow \]
\[C{{H}_{3}}C\equiv CH\xrightarrow[C{{u}_{2}}C{{l}_{2}}]{Ammoniacal}\underset{\operatorname{Re}d\,ppt}{\mathop{C{{H}_{3}}C\equiv CCu}}\,\downarrow \] 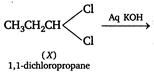
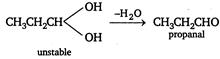
You need to login to perform this action.
You will be redirected in
3 sec
