A) benzyl alcohol and 2, 4, 6-tribromo-3- methoxy benzene
B) benzyl alcohol and 2, 4, 6-tribromo-3-methyl phenol
C) o-cresol and 3, 4, 5-tribromo-2-methyl phenol
D) methoxybenzene and 2, 4, 6-tribromo-3- methoxy benzene
E) m-cresol and 2, 4, 6-tribromo-3-methyl phenol
Correct Answer: E
Solution :
Compound X\[({{C}_{7}}{{H}_{8}}O)\]insoluble in aqueous \[NaHC{{O}_{3}}\]but soluble in\[NaOH,\]so it is a phenol. Since the number of carbon atoms remains the same after bromination, the compound must be meta cresol and reactions takes place as follows: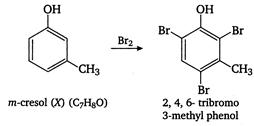
You need to login to perform this action.
You will be redirected in
3 sec
