A) \[2{{H}_{2}}S{{O}_{4}}+Cu\xrightarrow{{}}CuS{{O}_{4}}+2{{H}_{2}}O+S{{O}_{2}}\]
B) \[A{{s}_{2}}{{O}_{3}}+3{{H}_{2}}S\xrightarrow{{}}A{{s}_{2}}{{S}_{3}}+3{{H}_{2}}O\]
C) \[2KOH+C{{l}_{2}}\xrightarrow{{}}KCl+KOCl+{{H}_{2}}O\]
D) \[C{{a}_{3}}{{P}_{2}}+6{{H}_{2}}O\xrightarrow{{}}3Ca{{(OH)}_{2}}+2P{{H}_{3}}\]
E) \[4N{{H}_{3}}+3{{O}_{2}}\xrightarrow{{}}2{{N}_{2}}+6{{H}_{2}}O\]
Correct Answer: C
Solution :
The reactions, in which the same element is oxidized as well as reduced, are called disproportionation reactions.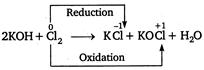 In this reaction, the same element, ie,\[C{{l}_{2}}\]is oxidized as well as reduced, so it is an example of disproportionation reaction.
In this reaction, the same element, ie,\[C{{l}_{2}}\]is oxidized as well as reduced, so it is an example of disproportionation reaction.
You need to login to perform this action.
You will be redirected in
3 sec
