A) tetrahedral and \[s{{p}^{3}}\]
B) square planar and\[ds{{p}^{2}}\]
C) square planar and \[s{{p}^{3}}{{d}^{2}}\]
D) octahedral and \[s{{p}^{3}}{{d}^{2}}\]
E) octahedral and \[{{d}^{2}}s{{p}^{3}}\]
Correct Answer: C
Solution :
In\[Xe{{F}_{4}},\]the central atom,\[Xe,\]has eight electrons in its outermost shell. Out of these four are used for forming four a bonds with F and four remain as lone pairs. \[\therefore \,Xe{{F}_{4}}\Rightarrow \,4\sigma \] bonds + 2 lone pairs \[\Rightarrow \]6 hybridized orbitals, ie,\[s{{p}^{3}}{{d}^{2}}\]hybridisation Since, two lone pairs of electrons are present, the geometry of \[Xe{{F}_{4}}\] becomes square planar from octahedral.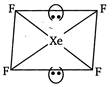
You need to login to perform this action.
You will be redirected in
3 sec
