A) aromatic nucleophilic substitution
B) aromatic electrophilic substitution
C) aromatic nucleophilic addition
D) aromatic electrophilic addition
E) free radical substitution
Correct Answer: B
Solution :
The chlorination of benzene in the presence of halogen carrier (ie, Lewis acid) is an example of aromatic electrophilic substitution. Mechanism of chlorination is as follows \[Cl-Cl+\underset{(ha\log en\text{ }carrier)}{\mathop{FeC{{l}_{3}}}}\,\xrightarrow{{}}\underset{(electrophile)}{\mathop{C{{l}^{+}}}}\,\]\[+\text{ }FeCl_{4}^{-}\]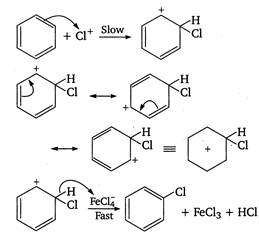
You need to login to perform this action.
You will be redirected in
3 sec
