A) \[{{H}_{2}}S\] is an acid and\[{{H}_{2}}{{O}_{2}}\]is a base
B) \[{{H}_{2}}S\]is a base and\[{{H}_{2}}{{O}_{2}}\]is an acid
C) \[{{H}_{2}}S\]is an oxidizing agent and\[{{H}_{2}}{{O}_{2}}\]is a reducing agent
D) \[{{H}_{2}}S\] is a reducing agent and\[{{H}_{2}}{{O}_{2}}\]is an oxidizing agent
E) \[{{H}_{2}}S\]is hydrolyzed to S
Correct Answer: D
Solution :
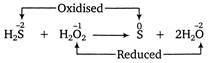 Here, the oxidation number of S increases from -2 in\[{{H}_{2}}S\]to 0 in elemental sulphur, while that of O decreases from -1 in\[{{H}_{2}}O\]to -2 in\[{{H}_{2}}O\],therefore\[{{H}_{2}}S\]is a reducing agent and \[{{H}_{2}}{{O}_{2}}\]is an oxidizing agent.
Here, the oxidation number of S increases from -2 in\[{{H}_{2}}S\]to 0 in elemental sulphur, while that of O decreases from -1 in\[{{H}_{2}}O\]to -2 in\[{{H}_{2}}O\],therefore\[{{H}_{2}}S\]is a reducing agent and \[{{H}_{2}}{{O}_{2}}\]is an oxidizing agent.
You need to login to perform this action.
You will be redirected in
3 sec
