A) 16.89
B) 32.22
C) 84.45
D) 28.15
E) 29.7
Correct Answer: A
Solution :
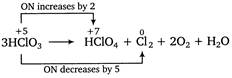 This is a disproportionation reaction since here the oxidation state of chlorine decreases from +5 to 0 in \[C{{l}_{2}}\] as well as increases from +5 to +7 in\[HCl{{O}_{4}}\]. Thus, \[HCl{{O}_{3}}\]acts as oxidizing as well as reducing agent. Equivalent mass of oxidizing agent (i.e.,\[HCl{{O}_{3}}\]) \[=\frac{molar\,mass}{Change\,in\,oxidation\,number}\] \[=\frac{84.45}{(5-0)}=16.89\]
This is a disproportionation reaction since here the oxidation state of chlorine decreases from +5 to 0 in \[C{{l}_{2}}\] as well as increases from +5 to +7 in\[HCl{{O}_{4}}\]. Thus, \[HCl{{O}_{3}}\]acts as oxidizing as well as reducing agent. Equivalent mass of oxidizing agent (i.e.,\[HCl{{O}_{3}}\]) \[=\frac{molar\,mass}{Change\,in\,oxidation\,number}\] \[=\frac{84.45}{(5-0)}=16.89\]
You need to login to perform this action.
You will be redirected in
3 sec
