A) \[{{[Co{{F}_{6}}]}^{3-}}\]
B) \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
C) \[{{[Fe{{(CN)}_{6}}]}^{3-}}\]
D) \[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
Correct Answer: A
Solution :
Electronic configuration of \[C{{O}^{2+}}\] ion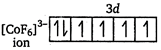
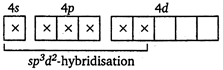 \[{{F}^{-}}\] is a weak ligand. It cannot pair up electrons with d-subshell and forms outer orbital octahedral complex. \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\] ion
\[{{F}^{-}}\] is a weak ligand. It cannot pair up electrons with d-subshell and forms outer orbital octahedral complex. \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\] ion 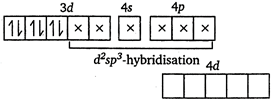
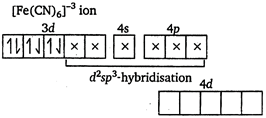
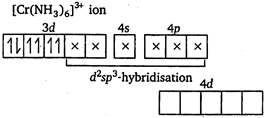 \[N{{H}_{3}}\] and \[C{{N}^{-}}\] are strong ligands. So, they form inner orbital complex.
\[N{{H}_{3}}\] and \[C{{N}^{-}}\] are strong ligands. So, they form inner orbital complex.
You need to login to perform this action.
You will be redirected in
3 sec
