A) free energy change for the formation of CO is more negative than that of \[F{{e}_{2}}{{O}_{3}}\]
B) CO is thermodynamically more stable than \[F{{e}_{2}}{{O}_{3}}\]
C) carbon has higher affinity towards oxygen than iron
D) iron has higher affinity towards oxygen than carbon
Correct Answer: D
Solution :
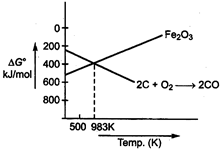 Below 983 K, conversion of iron to ferric oxide is more favourable.
Below 983 K, conversion of iron to ferric oxide is more favourable.
You need to login to perform this action.
You will be redirected in
3 sec
