A) \[1\]
B) \[2\]
C) \[0\]
D) \[3\]
Correct Answer: A
Solution :
In an antibonding molecular orbital, most of the electron density is located away from the space between the nuclei, as a result of which there is a nodal plane (i.e., a plane at which the electron density is zero) between the nuclei.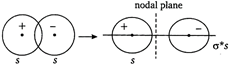
You need to login to perform this action.
You will be redirected in
3 sec
