A) \[{{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}}\]- Square planar
B) \[[Ni{{(CO)}_{4}}]\] - Neutral ligand
C) \[[Fe{{(C{{N}_{6}})}^{3-}}]\] - \[s{{p}^{3}}{{d}^{2}}\]
D) \[{{[Co{{(en)}_{3}}]}^{3+}}\] - Follows EAN rule
Correct Answer: C
Solution :
[a] In \[{{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}}\], \[Cu\] is present as \[C{{u}^{2+}}\] \[C{{u}^{2+}}=[Ar]3{{d}^{9}}\,4{{s}^{0}}\]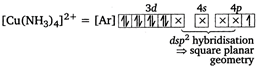 (\[N{{H}_{3}}\] being a strong field ligand shifts one electron from 3d orbital to 4p orbital.) [b] In \[[Ni{{(CO)}_{4}}],\]CO is a neutral ligand. [c] In \[{{[Fe{{(CN)}_{6}}]}^{3-}},\] Fe is present as \[F{{e}^{3+}}\] \[F{{e}^{3+}}=[Ar]3{{d}^{5}}4{{s}^{0}}\] \[{{[Fe{{(CN)}_{6}}]}^{3-}}=[Ar]\]
(\[N{{H}_{3}}\] being a strong field ligand shifts one electron from 3d orbital to 4p orbital.) [b] In \[[Ni{{(CO)}_{4}}],\]CO is a neutral ligand. [c] In \[{{[Fe{{(CN)}_{6}}]}^{3-}},\] Fe is present as \[F{{e}^{3+}}\] \[F{{e}^{3+}}=[Ar]3{{d}^{5}}4{{s}^{0}}\] \[{{[Fe{{(CN)}_{6}}]}^{3-}}=[Ar]\] You need to login to perform this action.
You will be redirected in
3 sec
