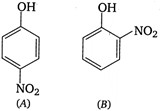
A) higher than that of A
B) lower than that of A
C) higher or lower than A depending on the size of the vessel
D) same as that of A
Correct Answer: A
Solution :
Vapour pressure of o-nitrophenol is more than vapour pressure of p-nitrophenol due to the intramolecular hydrogen bonding. As a result, it is more volatile.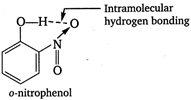
You need to login to perform this action.
You will be redirected in
3 sec
