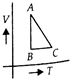
A) at A is more than at B
B) at C is less than at B
C) at B is more than at A
D) at A and B are equal
Correct Answer: D
Solution :
: The internal energy of an ideal gas is only dependent upon its temperature. From the graph, The ideal gas at same temperature at A and B. \[\therefore \]The internal at energy A and B are equal. or \[{{U}_{A}}={{U}_{B}}\]You need to login to perform this action.
You will be redirected in
3 sec
