A) \[C{{O}_{2}}\] is more stable than \[CO\] at less than 983 K
B) \[CO\] reduces \[F{{e}_{2}}{{O}_{3}}\] to Fe at less than 983 K
C) \[CO\] is less stable than \[C{{O}_{2}}\] at more than 983 K
D) \[CO\] reduces \[F{{e}_{2}}{{O}_{3}}\] to Fe in the reduction zone of Blast furnace
Correct Answer: C
Solution :
: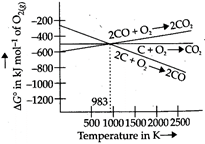 Ellingham diagram for the reducing nature of carbon
Ellingham diagram for the reducing nature of carbon 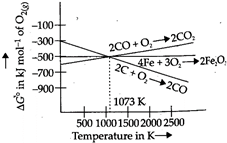 Ellingham diagram for the reduction of haematite Below \[983K,\text{ }\Delta {{G}^{o}}\] for the formation of \[C{{O}_{2}}\] is more negative than \[\Delta {{G}^{o}}\] for the formation of \[CO\], so \[C{{O}_{2}}\] is more stable. At temperature above \[983K\] \[\Delta {{G}^{o}}_{formation}\] of \[CO\] is more negative than \[\Delta {{G}^{o}}_{formation}\] of \[C{{O}_{2}}\], so \[CO\] is more stable. So statement is false. \[\Delta {{G}^{o}}_{formation}\] of \[C{{O}_{2}}\] from \[CO\] is more negative than \[\Delta {{G}^{o}}_{formation}\] of \[F{{e}_{2}}{{O}_{3}}\]. This means that \[F{{e}_{2}}{{O}_{3}}\] can be reduced by \[CO\] below\[1073K\].
Ellingham diagram for the reduction of haematite Below \[983K,\text{ }\Delta {{G}^{o}}\] for the formation of \[C{{O}_{2}}\] is more negative than \[\Delta {{G}^{o}}\] for the formation of \[CO\], so \[C{{O}_{2}}\] is more stable. At temperature above \[983K\] \[\Delta {{G}^{o}}_{formation}\] of \[CO\] is more negative than \[\Delta {{G}^{o}}_{formation}\] of \[C{{O}_{2}}\], so \[CO\] is more stable. So statement is false. \[\Delta {{G}^{o}}_{formation}\] of \[C{{O}_{2}}\] from \[CO\] is more negative than \[\Delta {{G}^{o}}_{formation}\] of \[F{{e}_{2}}{{O}_{3}}\]. This means that \[F{{e}_{2}}{{O}_{3}}\] can be reduced by \[CO\] below\[1073K\].
You need to login to perform this action.
You will be redirected in
3 sec
