A) \[\frac{1}{n},k\]
B) \[\log \frac{1}{n},k\]
C) \[\frac{1}{n},logk\]
D) \[\log \frac{1}{n},\,\log k\]
Correct Answer: C
Solution :
: Mathematically, Freundlich adsorption isotherm can be written as, \[\underbrace{\log \frac{x}{m}}_{y}=\underbrace{\operatorname{logk}}_{c}+\underbrace{\log \frac{1}{n}}_{m}\underbrace{\log \,p}_{x}\]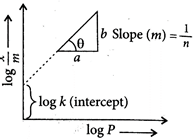
You need to login to perform this action.
You will be redirected in
3 sec
