A) \[M{{A}_{2}}{{B}_{2}}\]-Tetrahedral
B) \[MABCD\]-Tetrahedral
C) \[M{{A}_{3}}B\]-Square planar
D) \[MABCD\]-Square planar
Correct Answer: D
Solution :
: Tetrahedral complexes do not show geometrical isomerism because due to symmetrical structure, relative positions of the ligands is same with respect to each other. Square planar complexes of the type \[M{{A}_{3}}B\] do not show geometrical isomerism because the possible spatial arrangements are equivalent. MABCD type square planar complexes show three isomers which can be obtained by fixing the position of one ligand and placing any of the remaining three ligands at the trans position one by one.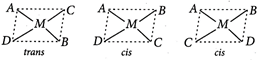
You need to login to perform this action.
You will be redirected in
3 sec
