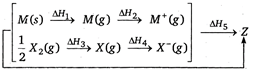question_answer 1) Solar spectrum is an example for
A)
line emission spectrum
done
clear
B)
continuous emission spectrum
done
clear
C)
band absorption spectrum
done
clear
D)
line absorption spectrum
done
clear
View Answer play_arrow
question_answer 2) When a piece of metal is illuminated by a monochromatic light of wavelength X, then stopping potential is\[3{{V}_{s}}\]b. When same surface is illuminated by light of wavelength 2/1, then stopping potential becomes\[{{V}_{s}}\]. The value of threshold wavelength for photoelectric emission will be
A)
\[4\lambda \]
done
clear
B)
\[8\lambda \]
done
clear
C)
\[\frac{4}{3}\lambda \]
done
clear
D)
\[6\lambda \]
done
clear
View Answer play_arrow
question_answer 3) The maximum kinetic energy of emitted electrons in a photoelectric effect does not depend upon
A)
wavelength
done
clear
B)
frequency
done
clear
C)
intensity
done
clear
D)
work function
done
clear
View Answer play_arrow
question_answer 4) The ratio of minimum wavelengths of Lyman and Balmer series will be
A)
1.25
done
clear
B)
0.25
done
clear
C)
5
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 5) Hydrogen atom does not emit -X-rays because
A)
it contains only a single electron
done
clear
B)
energy levels in it are far apart
done
clear
C)
its size is very small
done
clear
D)
energy levels in it are very dose to each other
done
clear
View Answer play_arrow
question_answer 6)
The potential difference between A and B in the following figure is
A)
32 V
done
clear
B)
48 V
done
clear
C)
24 V
done
clear
D)
14 V
done
clear
View Answer play_arrow
question_answer 7) The magnetic field at the centre of a circular current carrying conductor of radius r is B. The magnetic field on its axis at a distance r from the centre is Bg. The value of \[{{B}_{c}}:\text{ }{{B}_{a}}\]will be
A)
1 : \[\sqrt{2}\]
done
clear
B)
1 : 2\[\sqrt{2}\]
done
clear
C)
2\[\sqrt{2}\] : 1
done
clear
D)
\[\sqrt{2}\] : 1
done
clear
View Answer play_arrow
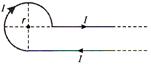
question_answer 8)
Current I is flowing in conductor shaped as shown in the figure. The radius of the curved part is r and the length of straight portion is very large. The value of the magnetic field at the centre O will be
A)
\[\frac{{{\mu }_{0}}I}{4\pi r}\left( \frac{3\pi }{2}+1 \right)\]
done
clear
B)
\[\frac{{{\mu }_{0}}I}{4\pi r}\left( \frac{3\pi }{2}-1 \right)\]
done
clear
C)
\[\frac{{{\mu }_{0}}I}{4\pi r}\left( \frac{\pi }{2}+1 \right)\]
done
clear
D)
\[\frac{{{\mu }_{0}}I}{4\pi r}\left( \frac{\pi }{2}-1 \right)\]
done
clear
View Answer play_arrow
question_answer 9) Two tangent galvanometers A and B are identical except in their number of turns. They are connected in series. On passing a current through them, deflections of \[60{}^\circ \] and \[30{}^\circ \]are produced. The ratio of the number of turns in A and B is
A)
1 : 3
done
clear
B)
3 : 1
done
clear
C)
1 : 2
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 10)
The resultant force on the current loop PQRS due to a long current carrying conductor will be
A)
\[{{10}^{-4}}N\]
done
clear
B)
\[3.6\times {{10}^{-4}}N\]
done
clear
C)
\[1.8\times {{10}^{-4}}N\]
done
clear
D)
\[5\times {{10}^{-4}}N\]
done
clear
View Answer play_arrow
question_answer 11) How many 6\[\mu \]F, 200 V condensers are needed to make a condenser of 18 \[\mu \]F, 600 V?
A)
9
done
clear
B)
18
done
clear
C)
3
done
clear
D)
27
done
clear
View Answer play_arrow
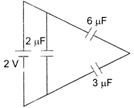
question_answer 12)
The total energy stored in the condenser system shown in the figure will be
A)
2\[\mu \]J
done
clear
B)
4\[\mu \]J
done
clear
C)
8\[\mu \]J
done
clear
D)
16\[\mu \]J
done
clear
View Answer play_arrow
question_answer 13) A metal wire is subjected to a constant potential difference. When the temperature of the metal wire increases, the drift velocity of the electron ink
A)
increases, thermal velocity of the electron decreases
done
clear
B)
decreases, thermal velocity of the electron decreases
done
clear
C)
increases, thermal velocity of the electron increases
done
clear
D)
decreases, thermal velocity of the electron increases
done
clear
View Answer play_arrow
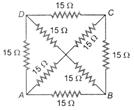
question_answer 14)
The equivalent resistance between the points A and B will be (each resistance is 15\[\Omega \])
A)
30\[\Omega \]
done
clear
B)
8\[\Omega \]
done
clear
C)
10\[\Omega \]
done
clear
D)
40\[\Omega \]
done
clear
View Answer play_arrow
question_answer 15) In the Bohr model of hydrogen atom, the electron is pictured to rotate in a circular orbit of radius\[5\times {{10}^{-11}}m\], at a speed \[2.2\times {{10}^{6}}\]m/s. What is the current associated with electron motion?
A)
1.12 mA
done
clear
B)
3 mA
done
clear
C)
0.75 mA
done
clear
D)
2.25 mA
done
clear
View Answer play_arrow
question_answer 16) A certain current on passing through a galvanometer produces a deflection of 100 divisions. When a shunt of one ohm is connected, the deflection reduces to 1 division. The galvanometer resistance is
A)
100\[\Omega \]
done
clear
B)
99\[\Omega \]
done
clear
C)
10\[\Omega \]
done
clear
D)
9.9\[\Omega \]
done
clear
View Answer play_arrow
question_answer 17) Two similar circular loops carry equal currents in the same direction. On moving coils further apart, the electric current will
A)
increase in both
done
clear
B)
decrease in both
done
clear
C)
remain unaltered
done
clear
D)
increases in one and decreases in the second
done
clear
View Answer play_arrow
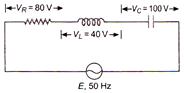
question_answer 18)
The value of alternating emf E in the given circuit will be
A)
220 V
done
clear
B)
140 V
done
clear
C)
100 V
done
clear
D)
20 V
done
clear
View Answer play_arrow
question_answer 19) A current of 5 A is flowing at 220 V in the primary coil of a transformer. If the voltage produced in the secondary coil is 2200 V and 50% of power is lost, then the current in secondary will be
A)
2.5 A
done
clear
B)
5 A
done
clear
C)
0.25 A
done
clear
D)
0.5 A
done
clear
View Answer play_arrow
question_answer 20) For a series LCR circuit at resonance, the statement which is not true is
A)
Peak energy stored by a capacitor = peak energy stored by an inductor.
done
clear
B)
Average power = apparent power.
done
clear
C)
Wattless current is zero.
done
clear
D)
Power factor is zero.
done
clear
View Answer play_arrow
question_answer 21) If \[{{\mu }_{0}}\]permeability of free space and \[{{\varepsilon }_{0}}\] is permittivity of free space, the speed of light in vacuum is given by
A)
\[\sqrt{{{\mu }_{0}}{{\varepsilon }_{0}}}\]
done
clear
B)
\[\sqrt{\frac{{{\mu }_{0}}}{{{\varepsilon }_{0}}}}\]
done
clear
C)
\[\sqrt{\frac{1}{{{\mu }_{0}}{{\varepsilon }_{0}}}}\]
done
clear
D)
\[\sqrt{\frac{{{\varepsilon }_{0}}}{{{\mu }_{0}}}}\]
done
clear
View Answer play_arrow
question_answer 22) In Youngs double slit experiment, a third slit is made in between the double slits. Then
A)
intensity of fringes totally disappears
done
clear
B)
only bright light is observed on the screen
done
clear
C)
fringes of unequal width are formed
done
clear
D)
contrast between bright and dark fringes is reduced
done
clear
View Answer play_arrow
question_answer 23) In a two slit experiment with monochromatic light fringes are obtained on a screen placed at some distance from the slits. If the screen is moved by \[5\times {{10}^{-2}}\]m towards the slits, the change in fringe width is\[3\times {{10}^{-5}}m\]. If separation between the slits is\[{{10}^{-3}}m\], the wavelength of light used is
A)
\[6000{{A}^{o}}\]
done
clear
B)
\[5000{{A}^{o}}\]
done
clear
C)
\[3000{{A}^{o}}\]
done
clear
D)
\[4500{{A}^{o}}\]
done
clear
View Answer play_arrow
question_answer 24) In a Fraunhofer diffraction experiment at a single slit using a light of wavelength 400 nm, the first minimum is formed at an angle of 30°. The direction 6 of the first secondary maximum is given by
A)
\[{{\sin }^{-1}}\left( \frac{2}{3} \right)\]
done
clear
B)
\[{{\sin }^{-1}}\left( \frac{3}{4} \right)\]
done
clear
C)
\[{{\sin }^{-1}}\left( \frac{1}{4} \right)\]
done
clear
D)
\[ta{{n}^{-1}}\left( \frac{2}{3} \right)\]
done
clear
View Answer play_arrow
question_answer 25) Maximum diffraction takes place in a given slit for
A)
\[\gamma \]-rays
done
clear
B)
ultraviolet light
done
clear
C)
infrared light
done
clear
D)
radiowaves
done
clear
View Answer play_arrow
question_answer 26) If an electron and a proton have the same de-Broglie wavelength, then the kinetic energy of the electron is
A)
zero
done
clear
B)
less than that of a proton
done
clear
C)
more than that of a proton
done
clear
D)
equal to that of a proton
done
clear
View Answer play_arrow
question_answer 27) Two protons are kept at a separation of\[40{{A}^{o}}\]. \[{{F}_{n}}\] is the nuclear force and \[{{F}_{e}}\]is the electrostatic force between them. Then
A)
\[{{F}_{n}}>>{{F}_{e}}\]
done
clear
B)
\[{{F}_{n}}={{F}_{e}}\]
done
clear
C)
\[{{F}_{n}}<<{{F}_{e}}\]
done
clear
D)
\[{{F}_{n}}={{F}_{e}}\]
done
clear
View Answer play_arrow
question_answer 28) Blue colour of sea water is due to
A)
interference of sunlight reflected from the water surface
done
clear
B)
scattering of sunlight by the water molecules
done
clear
C)
image of sky in water
done
clear
D)
refraction of sunlight
done
clear
View Answer play_arrow
question_answer 29) The ratio of the nuclear radii of elements with mass numbers 216 and 125 is
A)
216 : 125
done
clear
B)
\[\sqrt{216}:\sqrt{125}\]
done
clear
C)
6 : 5
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 30) On bombarding \[{{U}^{235}}\]by slow neutron, 200 MeV energy is released. If the power output of atomic reactor is 1.6 MW, then the rate of fission will be
A)
\[5\times {{10}^{22}}/s\]
done
clear
B)
\[5\times {{10}^{16}}/s\]
done
clear
C)
\[8\times {{10}^{16}}/s\]
done
clear
D)
\[20\times {{10}^{16}}/s\]
done
clear
View Answer play_arrow
question_answer 31) A ray of light enters from a rarer to a denser medium. The angle of incidence is i. Then the reflected and refracted rays are mutually perpendicular to each other. The critical angle for the pair of media is
A)
\[{{\sin }^{-1}}\left( \tan \,i \right)\]
done
clear
B)
\[ta{{n}^{-1}}\left( sin\,i \right)\]
done
clear
C)
\[si{{n}^{-1}}\left( \cot \,i \right)\]
done
clear
D)
\[{{\cos }^{-1}}\left( \tan \,i \right)\]
done
clear
View Answer play_arrow
question_answer 32) A fish in water (refractive index n) looks at a bird vertically above in the air. If y is the height of the bird and x is the depth of the fish from the surface, then the distance of the bird as estimated by the fish is
A)
\[x+y\left( 1-\frac{1}{n} \right)\]
done
clear
B)
\[x+ny\]
done
clear
C)
\[x+y\left( 1+\frac{1}{n} \right)\]
done
clear
D)
\[y+x\left( 1-\frac{1}{n} \right)\]
done
clear
View Answer play_arrow
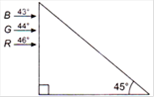
question_answer 33)
Figure shows a mixture of blue, green and red colored rays incident normally on a right angled prism. The critical angles of the material of the prism for red, green and blue are\[46{}^\circ \], \[44{}^\circ \] and \[43{}^\circ \]respectively. The arrangement will separate
A)
red colour from blue and green
done
clear
B)
blue colour from red and green
done
clear
C)
green colour from red and blue
done
clear
D)
all the three colours
done
clear
View Answer play_arrow
question_answer 34) A convex and a concave lens separated by distance d are then put in contact. The focal length of the combination
A)
decreases
done
clear
B)
increases
done
clear
C)
becomes 0
done
clear
D)
remains the same
done
clear
View Answer play_arrow
question_answer 35)
A convex lens is made of 3 layers of glass of 3 different materials as in the figures. A point object is placed on its axis. The number of images of the object are
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 36) An unpolarised beam of intensity l0 falls on a Polaroid. The intensity of the emergent light is
A)
\[\frac{{{I}_{0}}}{2}\]
done
clear
B)
\[{{I}_{0}}\]
done
clear
C)
\[\frac{{{I}_{0}}}{4}\]
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 37) Which of the following as a dichroic crystal?
A)
Quartz
done
clear
B)
Tourmaline
done
clear
C)
Mica
done
clear
D)
Selenite
done
clear
View Answer play_arrow
question_answer 38) Two identical metal spheres charged with \[+12\mu F\]and \[-8\mu F\]are kept at certain distance in air. They are brought into contact and then kept at the same distance. The ratio of the magnitudes of electrostatic forces between them before and after contact is
A)
12 : 1
done
clear
B)
8 : 1
done
clear
C)
24 : 1
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 39) A small conducting sphere of radius r is lying concentrically inside a bigger hollow conducting sphere of radius R. The bigger and smaller spheres are charged with Q and q (Q > q)and are insulated from each other. The potential difference between the spheres will be
A)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\left( \frac{q}{r}-\frac{q}{R} \right)\]
done
clear
B)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\left( \frac{q}{R}-\frac{Q}{r} \right)\]
done
clear
C)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\left( \frac{q}{r}-\frac{Q}{R} \right)\]
done
clear
D)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}\left( \frac{Q}{R}-\frac{q}{r} \right)\]
done
clear
View Answer play_arrow
question_answer 40) The charges Q, \[+q\]and \[+q\] are placed at the vertices of an equilateral triangle of side L If the net electrostatic potential energy of the system is zero, then Q is equal to
A)
\[-\frac{q}{2}\]
done
clear
B)
\[-q\]
done
clear
C)
\[\frac{+q}{2}\]
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 41) Dimensional formula for the universal gravitational constant G is
A)
\[\left[ {{M}^{-1}}{{L}^{2}}{{T}^{-2}} \right]~\]
done
clear
B)
\[\left[ {{M}^{0}}{{L}^{0}}{{T}^{0}} \right]\]
done
clear
C)
\[\left[ {{M}^{-1}}{{L}^{3}}{{T}^{-2}} \right]~\]
done
clear
D)
\[\left[ {{M}^{-1}}{{L}^{3}}{{T}^{-1}} \right]\]
done
clear
View Answer play_arrow
question_answer 42) A body is projected vertically upwards. The times corresponding to height h while ascending and while descending are \[{{t}_{1}}\]and \[{{t}_{2}}\]respectively. Then the velocity of projection is (g is acceleration due to gravity)
A)
\[g\sqrt{{{t}_{1}}{{t}_{2}}}\]
done
clear
B)
\[\frac{g{{t}_{1}}{{t}_{2}}}{{{t}_{1}}+{{t}_{2}}}\]
done
clear
C)
\[\frac{g\sqrt{{{t}_{1}}{{t}_{2}}}}{2}\]
done
clear
D)
\[\frac{g\left( {{t}_{1}}+{{t}_{2}} \right)}{2}\]
done
clear
View Answer play_arrow
question_answer 43) A mass of 10 kg is suspended from a spring balance. It is pulled aside by a horizontal string so that it makes an angle of \[60{}^\circ \]with the vertical. The new reading of the balance is
A)
20 kg-wt
done
clear
B)
10 kg-wt
done
clear
C)
10\[\sqrt{3}\]kg-wt
done
clear
D)
20\[\sqrt{3}\] kg-wt
done
clear
View Answer play_arrow
question_answer 44) A body weighs 50 g in air and 40 g in water. How much would it weigh in a liquid of specific gravity 1.5?
A)
30 g
done
clear
B)
35 g
done
clear
C)
65 g
done
clear
D)
45 g
done
clear
View Answer play_arrow
question_answer 45) A body of mass 4 kg is accelerated upon by a constant force, travels a distance of 5 m in the first second and a distance of 2 m in the third second. The force acting on the body is
A)
2 N
done
clear
B)
4 N
done
clear
C)
6 N
done
clear
D)
8 N
done
clear
View Answer play_arrow
question_answer 46) A simple pendulum is suspended from the ceiling of a lift. When the lift is at rest its time period is T. With what acceleration should the lift be accelerated upwards in order to reduce its period to T/2? (\[g\]is acceleration due to gravity).
A)
2 g
done
clear
B)
3 g
done
clear
C)
4 g
done
clear
D)
g
done
clear
View Answer play_arrow
question_answer 47) If y is the ratio of specific heats and R is the universal gas constant, then the molar specific heat at constant volume \[{{C}_{v}}\]is given by
A)
\[\gamma R\]
done
clear
B)
\[\frac{\left( \gamma -1 \right)R}{\gamma }\]
done
clear
C)
\[\frac{R}{\gamma -1}\]
done
clear
D)
\[\frac{\gamma R}{\gamma -1}\]
done
clear
View Answer play_arrow
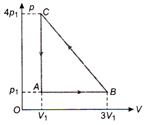
question_answer 48)
An ideal gas is taken via path ABCA as shown in figure. The net work done in the whole cycle is
A)
\[3{{p}_{1}}{{V}_{1}}\]
done
clear
B)
\[-3{{p}_{1}}{{V}_{1}}\]
done
clear
C)
\[6{{p}_{1}}{{V}_{1}}\]
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 49) In which of the processes, does the internal energy of the system remain constant?
A)
Adiabatic
done
clear
B)
Isochoric
done
clear
C)
Isobaric
done
clear
D)
Isothermal
done
clear
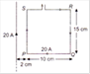
View Answer play_arrow
question_answer 50)
The coefficient of thermal conductivity of copper is 9 times that of steel. In the composite cylindrical bar shown in the figure, what will be the temperature at the junction of copper and steel?
A)
\[75{}^\circ C~\]
done
clear
B)
\[67{}^\circ C\]
done
clear
C)
\[25{}^\circ C~\]
done
clear
D)
\[33{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 51) The equation of a simple harmonic wave is given by \[y=6\]sin\[2\pi \]\[(2t-0.1\text{ }x)\], where x and y are in mm and t is in seconds. The phase difference between two particles 2 mm apart at any instant is
A)
\[18{}^\circ \]
done
clear
B)
\[36{}^\circ \]
done
clear
C)
\[54{}^\circ \]
done
clear
D)
\[72{}^\circ \]
done
clear
View Answer play_arrow
question_answer 52) With what velocity should an observer approach a stationary sound source, so that the apparent frequency of sound should appear double the actual frequency? (v is velocity of sound).
A)
\[\frac{v}{2}\]
done
clear
B)
\[3v\]
done
clear
C)
\[2v\]
done
clear
D)
\[v\]
done
clear
View Answer play_arrow
question_answer 53) If a black body emits 0.5 J of energy per second when it is at\[27{}^\circ C\], then the amount of energy emitted by it when it is at \[627{}^\circ C\]will be
A)
40.5 J
done
clear
B)
162 J
done
clear
C)
13.5 J
done
clear
D)
135 J
done
clear
View Answer play_arrow
question_answer 54) A string vibrates with a frequency of 200 Hz. When its length is doubled and tension is altered, it begins to vibrate with a frequency of 300 Hz. The ratio of the new tension to the original tension is
A)
9 : 1
done
clear
B)
1 : 9
done
clear
C)
3 : 1
done
clear
D)
1 : 3
done
clear
View Answer play_arrow
question_answer 55) How many times more intense is a 60 dB sound than a 30 dB sound?
A)
1000
done
clear
B)
2
done
clear
C)
100
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 56) The masses of two radioactive substances are same and their half-lives are 1 yr and 2 yr respectively. The ratio of their activities after 4 yr will be
A)
1 : 4
done
clear
B)
1 : 2
done
clear
C)
1 : 3
done
clear
D)
1 : 6
done
clear
View Answer play_arrow
question_answer 57) \[_{92}{{u}^{235}}\]Undergoes successive disintegrations with the end product of\[_{82}P{{b}^{203}}\]. The number of \[\alpha \] and \[\beta \] particles emitted are
A)
a = 6, p = 4
done
clear
B)
a = 6, p = 0
done
clear
C)
a = 8, p = 6
done
clear
D)
a = 3, p = 3
done
clear
View Answer play_arrow
question_answer 58) The most stable particle in Baryon group is
A)
neutron
done
clear
B)
omega-particle
done
clear
C)
proton
done
clear
D)
lambda-particle
done
clear
View Answer play_arrow
question_answer 59) In an unbiased p-n junction
A)
Potential at p is more than that at n
done
clear
B)
Potential at p is less than that at n
done
clear
C)
Potential at p is equal to that at n
done
clear
D)
Potential at p is +ve and that at n is -ve
done
clear
View Answer play_arrow
question_answer 60)
To get an output Y = 1 from the circuit shown, the inputs A, B and C must be respectively
A)
0, 1, 0
done
clear
B)
1, 0, 0
done
clear
C)
1, 0, 1
done
clear
D)
1, 1, 0
done
clear
View Answer play_arrow
question_answer 61) The number of nodal planes present in \[{{\sigma }^{*}}s\] antibonding orbitals is
A)
\[1\]
done
clear
B)
\[2\]
done
clear
C)
\[0\]
done
clear
D)
\[3\]
done
clear
View Answer play_arrow
question_answer 62) Which of the following electrolytic solutions has the least specific conductance?
A)
\[0.02N\]
done
clear
B)
\[0.2N\]
done
clear
C)
\[2N\]
done
clear
D)
\[0.002N\]
done
clear
View Answer play_arrow
question_answer 63) The overlapping of orbitals in benzene is of the type
A)
\[sp-sp\]
done
clear
B)
\[p-p\]
done
clear
C)
\[s{{p}^{2}}-s{{p}^{2}}\]
done
clear
D)
\[s{{p}^{3}}-s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 64) The calculated bond order of superoxide ion \[(O_{2}^{-})\] is
A)
\[2.5\]
done
clear
B)
\[2\]
done
clear
C)
\[1.5\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 65) Which of the following can be measured by the Ostwald-Walker dynamic method?
A)
Relative lowering of vapour pressure
done
clear
B)
Lowering of vapour pressure
done
clear
C)
Vapour pressure of the solvent
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 66) Mesomeric effect involves delocalisation of
A)
pi electrons
done
clear
B)
Sigma electrons
done
clear
C)
Protons
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 67) Which of the following has the maximum number of unpaired d electrons?
A)
\[Z{{n}^{2+}}\]
done
clear
B)
\[F{{e}^{2+}}\]
done
clear
C)
\[N{{i}^{3+}}\]
done
clear
D)
\[C{{u}^{+}}\]
done
clear
View Answer play_arrow
question_answer 68) One mole of which of the following has the highest entropy?
A)
Liquid nitrogen
done
clear
B)
Hydrogen gas
done
clear
C)
Mercury
done
clear
D)
Diamond
done
clear
View Answer play_arrow
question_answer 69) Which of the following species does not exert a resonance effect?
A)
\[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}\overset{+}{\mathop{N}}\,{{H}_{3}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}OH\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}Cl\]
done
clear
View Answer play_arrow
question_answer 70) A complex compound in which the oxidation number of a metal is zero is
A)
\[{{K}_{4}}[Fe{{(CN)}_{6}}]\]
done
clear
B)
\[{{K}_{3}}[Fe{{(CN)}_{6}}]\]
done
clear
C)
\[[Ni{{(CO)}_{4}}]\]
done
clear
D)
\[[Pt{{(N{{H}_{3}})}_{4}}]C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 71) Catalytic dehydrogenation of a primary alcohol gives a
A)
Secondary alcohol
done
clear
B)
Aldehyde
done
clear
C)
Ketone
done
clear
D)
Ester
done
clear
View Answer play_arrow
question_answer 72) Excess of \[PC{{l}_{5}}\] reacts with cone \[{{H}_{2}}S{{O}_{4}}\] giving
A)
Chlorosulphonic acid
done
clear
B)
Thionyl chloride
done
clear
C)
Sulphuryl chloride
done
clear
D)
Sulphurous acid
done
clear
View Answer play_arrow
question_answer 73) If one mole of ammonia and one mole of hydrogen chloride are mixed in a closed container to form ammonium chloride gas, then
A)
\[\Delta H>\Delta U\]
done
clear
B)
\[\Delta H=\Delta U\]
done
clear
C)
\[\Delta H<\Delta U\]
done
clear
D)
there is no relationship
done
clear
View Answer play_arrow
question_answer 74) The compound on dehydrogenation gives a ketone. The original compound is
A)
Primary alcohol
done
clear
B)
Secondary alcohol
done
clear
C)
Tertiary alcohol
done
clear
D)
Carboxylic acid
done
clear
View Answer play_arrow
question_answer 75) Which is the most easily liquefiable rare gas?
A)
\[Xe\]
done
clear
B)
\[Kr\]
done
clear
C)
\[Ar\]
done
clear
D)
\[Ne\]
done
clear
View Answer play_arrow
question_answer 76) Three moles of \[PC{{l}_{5}}\], three moles of \[PC{{l}_{3}}\] and two moles of \[C{{l}_{2}}\] are taken in a closed vessel. If at equilibrium the vessel has 1.5 moles of \[PC{{l}_{5}}\], the number of moles of \[PC{{l}_{3}}\] present in it is
A)
\[5\]
done
clear
B)
\[3\]
done
clear
C)
\[6\]
done
clear
D)
\[4.5\]
done
clear
View Answer play_arrow
question_answer 77) How many optically active stereomers are possible for butan-2,3-diol?
A)
\[1\]
done
clear
B)
\[2\]
done
clear
C)
\[3\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 78) An octahedral complex is formed when hybrid orbitals of the following type are involved
A)
\[s{{p}^{3}}\]
done
clear
B)
\[ds{{p}^{2}}\]
done
clear
C)
\[{{d}^{2}}s{{p}^{3}}\]
done
clear
D)
\[s{{p}^{2}}{{d}^{2}}\]
done
clear
View Answer play_arrow
question_answer 79) For the reaction \[2HI(g)\rightleftharpoons {{H}_{2}}(g)+{{I}_{2}}(g)-Q\,kJ\],the equilibrium constant depends upon
A)
Temperature
done
clear
B)
Pressure
done
clear
C)
Catalyst
done
clear
D)
Volume
done
clear
View Answer play_arrow
question_answer 80) The angle strain in cyclobutane is
A)
\[{{24}^{o}}44\]
done
clear
B)
\[{{29}^{o}}16\]
done
clear
C)
\[{{19}^{o}}22\]
done
clear
D)
\[{{9}^{o}}44\]
done
clear
View Answer play_arrow
question_answer 81) Methoxy methane and ethanol are
A)
Position isomers
done
clear
B)
Chain isomers
done
clear
C)
Functional isomers
done
clear
D)
Optical isomers
done
clear
View Answer play_arrow
question_answer 82) When the azimuthal quantum number has the value of 2, the number of orbitals possible are
A)
\[7\]
done
clear
B)
\[5\]
done
clear
C)
\[3\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 83) For the reaction \[F{{e}_{2}}{{O}_{3}}+3CO\xrightarrow{{}}2Fe+3C{{O}_{2}},\]the volume of carbon monoxide required to reduce one mole of ferric oxide is
A)
\[22.4d{{m}^{3}}\]
done
clear
B)
\[44.8d{{m}^{3}}\]
done
clear
C)
\[67.2\text{ }d{{m}^{3}}\]
done
clear
D)
\[11.2\text{ }d{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 84) The monomers of buna-S rubber are
A)
Vinyl chloride and sulphur
done
clear
B)
Butadiene
done
clear
C)
Styrene and butadiene
done
clear
D)
Isoprene and butadiene
done
clear
View Answer play_arrow
question_answer 85) An element with atomic number 21 is a
A)
Halogen
done
clear
B)
Representative element
done
clear
C)
Transition element
done
clear
D)
Alkali metal
done
clear
View Answer play_arrow
question_answer 86) n-propyl bromide on treating with alcoholic \[KOH\] produces
A)
Propane
done
clear
B)
propene
done
clear
C)
Propyne
done
clear
D)
propanol
done
clear
View Answer play_arrow
question_answer 87) Mercury is a liquid metal because
A)
It has a completely filled s orbital
done
clear
B)
It has a small atomic size
done
clear
C)
It has a completely filled d orbital that prevents d-d overlapping of orbitals
done
clear
D)
It has a completely filled d orbital that causes d-d overlapping
done
clear
View Answer play_arrow
question_answer 88) A compound is formed by elements A and B. This crystallises in the cubic structure where the A atoms are at the comers of the cube and B atoms are at the body centres. The simplest formula of the compound is
A)
\[AB\]
done
clear
B)
\[{{A}_{6}}B\]
done
clear
C)
\[{{A}_{8}}{{B}_{4}}\]
done
clear
D)
\[A{{B}_{6}}\]
done
clear
View Answer play_arrow
question_answer 89) Anisole can be prepared by the action of methyl iodide on sodium phenate. TW reaction is called
A)
Wurtzs reaction
done
clear
B)
Williamsons reaction
done
clear
C)
Fittigs reaction
done
clear
D)
Etards reaction
done
clear
View Answer play_arrow
question_answer 90) Malleability and ductility of metals can be accounted due to
A)
The presence of electrostatic force
done
clear
B)
The crystalline structure in metal
done
clear
C)
The capacity of layers of metal ions to slide over the other
done
clear
D)
the interaction of electrons with metal ions in the lattice
done
clear
View Answer play_arrow
question_answer 91) The correct order in which the first ionisation potential increases is
A)
\[Na,K,Be\]
done
clear
B)
\[K,Na,Be\]
done
clear
C)
\[K,Be,Na\]
done
clear
D)
\[Be,Na,K\]
done
clear
View Answer play_arrow
question_answer 92) \[10c{{m}^{3}}\]of \[0.1N\]monobasic acid requires \[15\text{ }c{{m}^{3}}\]of sodium hydroxide solution whose normality is
A)
\[1.5N\]
done
clear
B)
\[0.15N\]
done
clear
C)
\[0.066N\]
done
clear
D)
\[0.66N\]
done
clear
View Answer play_arrow
question_answer 93) The IUPAC name for tertiary butyl iodide is
A)
4-iodo butane
done
clear
B)
2-iodo butane
done
clear
C)
l-iodo-3-methyl propane
done
clear
D)
2-iodo-2-methyl propane
done
clear
View Answer play_arrow
question_answer 94) When sulphur dioxide is passed in an acidified \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\] solution, the oxidation state of sulphur is changed from
A)
\[+4\]to \[0\]
done
clear
B)
\[+4\] to \[+2\]
done
clear
C)
\[+4\] to \[+6\]
done
clear
D)
\[+6\] to \[+4\]
done
clear
View Answer play_arrow
question_answer 95) Mass of \[0.1\] mole of methane is
A)
\[1g\]
done
clear
B)
\[16g\]
done
clear
C)
\[1.6g\]
done
clear
D)
\[0.1g\]
done
clear
View Answer play_arrow
question_answer 96) The maximum number of hydrogen bonds that a molecule of water can have is
A)
\[1\]
done
clear
B)
\[2\]
done
clear
C)
\[3\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 97) A gas deviates from ideal behavior at a high pressure because its molecules
A)
Attract one another
done
clear
B)
show the Tyndall effect
done
clear
C)
have kinetic, energy
done
clear
D)
are bound by covalent bonds
done
clear
View Answer play_arrow
question_answer 98) The reagent used to convert an alkyne to alkene is
A)
\[Zn/HCl\]
done
clear
B)
\[Sn/HCl\]
done
clear
C)
\[Zn-Hg/HCl\]
done
clear
D)
\[Pd/{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 99) When compared to \[\Delta {{G}^{o}}\] for the formation of \[A{{l}_{2}}{{O}_{3}}\], the \[\Delta {{G}^{o}}\] for the formation of \[C{{r}_{2}}{{O}_{3}}\] is
A)
Higher
done
clear
B)
lower
done
clear
C)
Same
done
clear
D)
unpredicted
done
clear
View Answer play_arrow
question_answer 100) In order to increase the volume of a gas by 10%, the pressure of the gas should be
A)
Increased by 10%
done
clear
B)
Increased by 1%
done
clear
C)
Decreased by 10%
done
clear
D)
Decreased by 1%
done
clear
View Answer play_arrow
question_answer 101) Helium is used in balloons in place of hydrogen because it is
A)
Incombustible
done
clear
B)
Lighter than hydrogen
done
clear
C)
Radioactive
done
clear
D)
More abundant than hydrogen
done
clear
View Answer play_arrow
question_answer 102) The basic principle of Cottrells precipitator is
A)
Le-Chateliers principle
done
clear
B)
Peptisation
done
clear
C)
Neutralisation of charge on colloidal particles
done
clear
D)
Scattering of light
done
clear
View Answer play_arrow
question_answer 103) When carbon monoxide is passed over solid caustic soda heated to\[{{200}^{o}}C\], it forms
A)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
B)
\[NaHC{{O}_{3}}\]
done
clear
C)
\[HCOONa~\]
done
clear
D)
\[C{{H}_{3}}COONa\]
done
clear
View Answer play_arrow
question_answer 104) \[{{N}_{2}}+3{{H}_{2}}2N{{H}_{3}}+heat\]. What is the effect of the increase of temperature on the equilibrium of the reaction?
A)
Equilibrium is shifted to the left
done
clear
B)
Equilibrium is shifted to the right
done
clear
C)
Equilibrium is unaltered
done
clear
D)
Reaction rate does not change
done
clear
View Answer play_arrow
question_answer 105) Hydrogen gas is not liberated when the following metal is added to dil \[HCl\]
A)
\[Ag\]
done
clear
B)
\[Zn\]
done
clear
C)
\[Mg\]
done
clear
D)
\[Sn\]
done
clear
View Answer play_arrow
question_answer 106)
Consider the Born-Haber cycle for the formation of an ionic compound given below and identify the compound (Z) formed.
A)
\[{{M}^{+}}{{X}^{-}}\]
done
clear
B)
\[{{M}^{+}}{{X}^{-}}(s)\]
done
clear
C)
\[MX\]
done
clear
D)
\[{{M}^{+}}{{X}^{-}}(g)\]
done
clear
View Answer play_arrow
question_answer 107) In the brown ring test, the brown colour of the ring is due to
A)
Ferrous nitrate
done
clear
B)
Ferric nitrate
done
clear
C)
A mixture of \[NO\] and \[N{{O}_{2}}\]
done
clear
D)
Nitrosoferrous sulphate
done
clear
View Answer play_arrow
question_answer 108) Amines behave as
A)
Lewis acid
done
clear
B)
Lewis base
done
clear
C)
Aprotic acid
done
clear
D)
Neutral compound
done
clear
View Answer play_arrow
question_answer 109) Dalda is prepared from oils by
A)
Oxidation
done
clear
B)
Reduction
done
clear
C)
Hydrolysis
done
clear
D)
Distillation
done
clear
View Answer play_arrow
question_answer 110) The chemical name of anisole is
A)
Ethanoic acid
done
clear
B)
Methoxy benzene
done
clear
C)
Propanone
done
clear
D)
Acetone
done
clear
View Answer play_arrow
question_answer 111) The number of disulphide linkages present in insulin are
A)
\[1\]
done
clear
B)
\[2\]
done
clear
C)
\[3\]
done
clear
D)
\[4\]
done
clear
View Answer play_arrow
question_answer 112) 80 g of oxygen contains as many atoms as in
A)
80 g of hydrogen
done
clear
B)
1 g of hydrogen
done
clear
C)
10 g of hydrogen
done
clear
D)
5 g of hydrogen
done
clear
View Answer play_arrow
question_answer 113) Which metal has a greater tendency to form metal oxide?
A)
\[Cr\]
done
clear
B)
\[Fe\]
done
clear
C)
\[Al\]
done
clear
D)
\[Ca\]
done
clear
View Answer play_arrow
question_answer 114) Identify the reaction that does not take place in a blast furnace.
A)
\[CaC{{O}_{3}}\xrightarrow{{}}CaO+C{{O}_{2}}\]
done
clear
B)
\[CaO+Si{{O}_{2}}\xrightarrow{{}}CaSi{{O}_{3}}\]
done
clear
C)
\[2F{{e}_{2}}{{O}_{3}}+3C\xrightarrow{{}}4Fe+3C{{O}_{2}}\]
done
clear
D)
\[C{{O}_{2}}+C\xrightarrow{{}}2CO\]
done
clear
View Answer play_arrow
question_answer 115) Waxes are esters of
A)
Glycerol
done
clear
B)
Long chain alcohols
done
clear
C)
Glycerol and fatty acid
done
clear
D)
long chain alcohols and long chain fatty acids
done
clear
View Answer play_arrow
question_answer 116) An ionic compound is expected to have tetrahedral structure if \[{{r}_{+}}/{{r}_{-}}\] lies in the range of
A)
\[0.414\]to\[0.732\],
done
clear
B)
\[0.225\]to \[0.414\]
done
clear
C)
0.155 to \[0.225\]
done
clear
D)
\[0.732\] to \[1\]
done
clear
View Answer play_arrow
question_answer 117) Among the following, which is least acidic?
A)
Phenol
done
clear
B)
o-cresol
done
clear
C)
p-nitrophenol
done
clear
D)
p-chlorophenol
done
clear
View Answer play_arrow
question_answer 118) A ligand can also be regarded as
A)
Lewis acid
done
clear
B)
Bronsted base
done
clear
C)
Lewis base
done
clear
D)
Bronsted acid
done
clear
View Answer play_arrow
question_answer 119) The colour of sky is due to
A)
Transmission of light
done
clear
B)
Wavelength of scattered light
done
clear
C)
Absorption of light by atomspheric gases
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 120) Which of the following organic compounds answers to both iodoform test and Fehlings test?
A)
Ethanol
done
clear
B)
Methanal
done
clear
C)
Ethanal
done
clear
D)
Propanone
done
clear
View Answer play_arrow
question_answer 121) A leaf peeling of Tradescantia is kept in a medium having 10% NaCl. After a few minutes if we observe the leaf peel under the microscope, we are likely to see
A)
entry of water into the cell
done
clear
B)
the cells bursting out
done
clear
C)
diffusion of NaCl into the cell
done
clear
D)
exit of water from the cell
done
clear
View Answer play_arrow
question_answer 122) Early leaf spot disease in Arachis hypogea is caused due to infection of
A)
Circospora personata
done
clear
B)
Gibberella fujikuroi
done
clear
C)
Agrobacterium tumefaciens
done
clear
D)
Phytophthora infestans
done
clear
View Answer play_arrow
question_answer 123) Excessive growth of hair on the pinna is a feature found only in males because
A)
the female sex hormone estrogen suppresses the character in females
done
clear
B)
the gene responsible for the character is present on the Y - chromosome only
done
clear
C)
the gene responsible for the character is recessive in females and dominant only in males
done
clear
D)
the character is induced in males as males produce testosterone
done
clear
View Answer play_arrow
question_answer 124) Rapid increase in the blood sugar level of a patient can be immediately reduced by
A)
injecting insulin intravenously
done
clear
B)
injecting insulin intramuscularly
done
clear
C)
administering glucagon intravenously
done
clear
D)
consuming large quantities of insulin tablets
done
clear
View Answer play_arrow
question_answer 125) Which of the following is found exclusively in the sea water?
A)
Crabs
done
clear
B)
Oysters
done
clear
C)
Prawns
done
clear
D)
Trygon
done
clear
View Answer play_arrow
question_answer 126) The problem of necrosis and gradual senescence while performing tissue culture can be overcome by
A)
spraying auxins
done
clear
B)
spraying cytokinins
done
clear
C)
suspension culture
done
clear
D)
subculture
done
clear
View Answer play_arrow
question_answer 127) The immunoglobulin present in mothers milk is
A)
IgD
done
clear
B)
IgE
done
clear
C)
IgM
done
clear
D)
IgA
done
clear
View Answer play_arrow
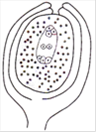
question_answer 128)
The diagram given by the side represents the sectional view of
A)
amphitropous ovule
done
clear
B)
campylotropous ovule
done
clear
C)
anatropous ovule
done
clear
D)
orthotropous ovule
done
clear
View Answer play_arrow
question_answer 129) In CAM plants, \[C{{O}_{2}}\]required for photosynthesis enters the plant body during
A)
day time through the lenticles
done
clear
B)
night through the stomata which are kept open
done
clear
C)
day time when the stomata are open
done
clear
D)
night when the hydathodes are open
done
clear
View Answer play_arrow
question_answer 130) A detritivorous animal of economic importance is
A)
earthworm
done
clear
B)
giriraja fowl
done
clear
C)
caterpillar larva
done
clear
D)
leech
done
clear
View Answer play_arrow
question_answer 131) Human egg is
A)
alecithal
done
clear
B)
centrolecithal
done
clear
C)
telolecithal
done
clear
D)
megalecithal
done
clear
View Answer play_arrow
question_answer 132) There are 64 codons in the genetic dictionary as
A)
there are 3 nonsense codons and 61 sense codons
done
clear
B)
there are 64 different types of tRNA
done
clear
C)
there are 64 amino acids to be coded
done
clear
D)
genetic code has a triplet nature
done
clear
View Answer play_arrow
question_answer 133) Vasopressin released from the neurohypophysis is mainly responsible for
A)
facultative reabsorption of water through Henles loop
done
clear
B)
obligatory reabsorption of water through Bowmans capsule
done
clear
C)
facultative reabsorption of water through DCT
done
clear
D)
obligatory reabsorption of water through PCT
done
clear
View Answer play_arrow
question_answer 134) Identify the substage of prophase - I of meiosis during which synapsis takes place
A)
diplotene
done
clear
B)
zygotene
done
clear
C)
leptotene
done
clear
D)
pachytene
done
clear
View Answer play_arrow
question_answer 135) Protein part of a holoenzyme is called
A)
exoenzyme
done
clear
B)
endoenzyme
done
clear
C)
coenzyme
done
clear
D)
apoenzyme
done
clear
View Answer play_arrow
question_answer 136) A gradual decrease in the size of the tail during metamorphosis in the life cycle of frog is a good example for
A)
programmed cell death
done
clear
B)
cell necrosis
done
clear
C)
cell senescence
done
clear
D)
pinocytic activity
done
clear
View Answer play_arrow
question_answer 137) Spot out the zone of our country considered as the Hot spot of biodiversity and regarded as the Cradle of Speciation.
A)
Western Ghats
done
clear
B)
North East
done
clear
C)
Himalayan base
done
clear
D)
Deccan Plateau
done
clear
View Answer play_arrow
question_answer 138) In Bt cotton, a transgenic plant, Bt refers to
A)
botanical
done
clear
B)
beta
done
clear
C)
biotechnology
done
clear
D)
Bacillus thurengiensis
done
clear
View Answer play_arrow
question_answer 139) Sequence of cellular layers from the periphery towards the cortex in an old dicot stem is
A)
epidermis, hypodermis, phellogen, phelloderm
done
clear
B)
epidermis, phellogen, phellum, epidermis
done
clear
C)
epidermis, hypodermis, cortex, endodermis
done
clear
D)
epidermis, phellum, phellogen, phelloderm
done
clear
View Answer play_arrow
question_answer 140) Higher frequency of melanine British moths and DDT resistance in mosquitoes are cited as examples for
A)
natural selection
done
clear
B)
point mutation
done
clear
C)
arrival of the fittest
done
clear
D)
genetic drift
done
clear
View Answer play_arrow
question_answer 141) Benedicts reagent test is conducted to confirm the presence of
A)
polysaccharides like starch
done
clear
B)
lipids
done
clear
C)
reducing sugars
done
clear
D)
proteins
done
clear
View Answer play_arrow
question_answer 142) An alkaloid called Reserpine is extracted from
A)
leaves of ashwagandha
done
clear
B)
roots of sarpagandha
done
clear
C)
leaves of sarpagandha
done
clear
D)
roots of ashwagandha
done
clear
View Answer play_arrow
question_answer 143) Nobel Prize for medicine was given for confirming the role of Helicobacter pylori in causing
A)
nephritis
done
clear
B)
rhinitis
done
clear
C)
bronchitis
done
clear
D)
peptic ulcer
done
clear
View Answer play_arrow
question_answer 144) In the sigmoid growth curve given by the side, the alphabets indicate the sequence of events. Choose the correct option where the alphabet specifies the event
A)
A = Phase of slow growth B = Phase of exponential growth C = Phase of diminishing growth D = Stationary phase
done
clear
B)
A = Phase of rapid growth B = Phase of diminishing growth C = Stationary phase D = Phase of slow growth
done
clear
C)
A = Diminishing growth B = Exponential growth C = Slow growth D = Stationary growth
done
clear
D)
A = Stationary phase B = Phase of slow growth C = Phase of rapid growth D = Phase of diminishing growth
done
clear
View Answer play_arrow
question_answer 145) Transformation of the early reducing atmosphere of the earth into an oxidizing atmosphere was mainly due to the activities of
A)
anaerobic photosynthesizers
done
clear
B)
anaerobic chemoheterotrophs
done
clear
C)
aerobic photosynthesizers
done
clear
D)
anaerobic heterotrophs
done
clear
View Answer play_arrow
question_answer 146) Genetically dwarf plants can be induced to grow tall by using
A)
gibberellins
done
clear
B)
phycobillins
done
clear
C)
auxins
done
clear
D)
cytokinins
done
clear
View Answer play_arrow
question_answer 147)
Match the phenomenon listed under Column - I with those listed under Column - II. Select the correct answer from the options given Column - I Column - II A. Warburg effect 1. Change in gene frequency by chance B. Pasteur effect 2. Postponing severance in the leaves by applying cytokinin C. Emerson effect 3. Decline in the consumption of respiratory substrate due to a change from anaerobic to aerobic respiration D. Wright effect 4. Inhibitory effect of \[{{O}_{2}}\]on photosynthesis ` 5. Enhancement of photosynthesis by subjecting chlorophyll to the effect of two different wavelengths of light
A)
A- 4 B-5 C-2 D-3
done
clear
B)
A-5 B-3 C-1 D-4
done
clear
C)
A- 5 B-4 C-1 D-2
done
clear
D)
A-4 B- 3 C-5 D-1
done
clear
View Answer play_arrow
question_answer 148) Which of the following is an agrostologic method of soil conservation?
A)
Basin listing
done
clear
B)
Terracing
done
clear
C)
Dry farming
done
clear
D)
Mulching
done
clear
View Answer play_arrow
question_answer 149) The sequence of events mentioned below are symbolised by alphabets. Choose the correct answer where the alphabets are matched with the processes \[\begin{align} & RNA\xrightarrow[{}]{A}DNA\xrightarrow[{}]{B}DNA \\ & \xrightarrow[{}]{{}}mRNA\xrightarrow[{}]{D}Polypeptide \\ \end{align}\]
A)
A = Replication B = Transformation C = Transcription D = Translation
done
clear
B)
A = Reverse transcription B = Replication C = Transcription D = Translation
done
clear
C)
A = Replication B = Transcription C = Translation D = Transduction
done
clear
D)
A = Reverse transcription B = Translation C = Transcription D = Replication
done
clear
View Answer play_arrow
question_answer 150) Identify the alga known for a biological activity called bioluminescence
A)
Spirogyra
done
clear
B)
Chlorella
done
clear
C)
Cydotella
done
clear
D)
Noctiluca
done
clear
View Answer play_arrow
question_answer 151) Who among the following is recognized as the father of Immunology?
A)
Robert Koch
done
clear
B)
Ferdinand Kohn
done
clear
C)
Edward Jenner
done
clear
D)
Louis Pasteur
done
clear
View Answer play_arrow
question_answer 152) Whether a child died after normal birth or died before birth can be confirmed by measuring
A)
tidal volume of air
done
clear
B)
residual volume of air
done
clear
C)
the weight of the child
done
clear
D)
the dead space air
done
clear
View Answer play_arrow
question_answer 153) Syndactyly, prehensile tail and long protrusible tongue are the unique features of
A)
rhesus monkey
done
clear
B)
Archaeopteryx
done
clear
C)
horse fish
done
clear
D)
Chaemeleon
done
clear
View Answer play_arrow
question_answer 154)
Match the hormones listed under Column - I with their functions listed under Column - II. Choose the answer which gives the correct combination of the alphabets of the two columns. Column-I Column-II A Oxytocin 1. Stimulates ovulation B Prolactin 2. Implantation and maintenance of pregnancy C Luteinizing hormone 3. Lactation after child birth D Progesteron 4. 5. Uterine contraction during labour Reabsorption of water by nephrons
A)
A- 4 B-2 C- 3 D- 5
done
clear
B)
A-5 B-3 C-1 D-4
done
clear
C)
A-4 B-3 C-1 D-2
done
clear
D)
A- 5 B-2 C-4 D-3
done
clear
View Answer play_arrow
question_answer 155) A dihybrid test cross-yielding a result of 1 : 1 : 1 : 1 ratio is indicative of
A)
4 different types of gametes produced by the \[{{F}_{1}}\]- dihybrid
done
clear
B)
homozygous condition of the \[{{F}_{1}}\]- dihybrid
done
clear
C)
4 different types of \[{{F}_{1}}\]- generation dihybrids
done
clear
D)
4 different types of gametes produced by the \[{{P}_{1}}\]- parent
done
clear
View Answer play_arrow
question_answer 156) Intercalary meristem is a derivative of
A)
lateral meristem
done
clear
B)
promeristem
done
clear
C)
primary meristem
done
clear
D)
secondary meristem
done
clear
View Answer play_arrow
question_answer 157) Which one of the following is not a device to promote cross - pollination?
A)
Cleistogamy
done
clear
B)
Heterostyly
done
clear
C)
Herkogamy
done
clear
D)
Dichogamy
done
clear
View Answer play_arrow
question_answer 158) Anterior choroid plexus is present on the
A)
floor of diencephalon
done
clear
B)
cerebral hemispheres
done
clear
C)
roof of diencephalon
done
clear
D)
roof of medulla oblongata
done
clear
View Answer play_arrow
question_answer 159) Which of the following statement is true about viruses?
A)
All viruses known to man are obligate parasites
done
clear
B)
Some viruses have cellular structure and are saprophytes
done
clear
C)
Viruses are filterable facultative parasites
done
clear
D)
Viruses are capable of performing metabolic activities on their own
done
clear
View Answer play_arrow
question_answer 160) A condition where a certain gene is present in only a single copy in a diploid cell is called
A)
heterozygous
done
clear
B)
monogamous
done
clear
C)
homozygous
done
clear
D)
hemizygous
done
clear
View Answer play_arrow
question_answer 161) An autosomal genetic disorder called cri-du-chat is caused due to
A)
non - disjunction
done
clear
B)
mutation
done
clear
C)
deletion
done
clear
D)
duplication
done
clear
View Answer play_arrow
question_answer 162) Notochord, skeletal system and dermis of the skin are the derivatives of
A)
mesoderm
done
clear
B)
endoderm
done
clear
C)
ectoderm
done
clear
D)
all the three layers
done
clear
View Answer play_arrow
question_answer 163) Photosynthesis cannot continue for long if during light reaction, only cyclic photophosphorylation takes place. This is because
A)
only ATP is formed \[NADP{{H}^{+}}+\text{ }{{H}^{+}}\]is not formed
done
clear
B)
photosystem-I stops getting excited at a wavelength of light beyond 680 nm
done
clear
C)
there is unidirectional cyclic movement of the electrons
done
clear
D)
there is no evolution of\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 164) Which of the following sequence is truely a systemic circulation pathway?
A)
Right ventricle \[\to \] Pulmonary aorta \[\to \] Tissues \[\to \] Pulmonary veins \[\to \] Left auricle
done
clear
B)
Right auricle \[\to \] Left ventricle \[\to \] aorta \[\to \] Tissues \[\to \] Veins \[\to \] Right auricle
done
clear
C)
Left auricle \[\to \] Left ventricle \[\to \] pulmonary aorta \[\to \] Tissues \[\to \] Right auricle
done
clear
D)
Left auricle \[\to \] Left ventricle \[\to \] aorta \[\to \] Arteries \[\to \] Tissues \[\to \] veins \[\to \] Right atrium
done
clear
View Answer play_arrow
question_answer 165) Oxalosuccinic acid, an intermediary compound of Krebs cycle is a
A)
5 carbon compound
done
clear
B)
6 carbon compound
done
clear
C)
4 carbon compound
done
clear
D)
3 carbon compound
done
clear
View Answer play_arrow
question_answer 166) When body tissues are injured resulting in the loss of blood, the process of blood clotting begins and the blood platelets release
A)
fibrinogen
done
clear
B)
thromboplastin
done
clear
C)
prothrombin
done
clear
D)
thrombin
done
clear
View Answer play_arrow
question_answer 167) According to the tac - operon concept, which functional unit of the bacterial genetic material is responsible for suppressing the activity of the operator gene in the absence of lactose?
A)
regulator gene
done
clear
B)
structural gene
done
clear
C)
promoter gene
done
clear
D)
represser protein
done
clear
View Answer play_arrow
question_answer 168) A hybrid where the cytoplasm of two parent cells are fused by retaining only one parental nucleus is called
A)
asymmetric somatic hybrid
done
clear
B)
cybrid
done
clear
C)
an interbreed
done
clear
D)
symmetric somatic hybrid
done
clear
View Answer play_arrow
question_answer 169) A sexually transmitted disease symptomized by the development of chancre on the genitals is caused by the infection of
A)
Treponema pallidum
done
clear
B)
Neisseria gonorrhoeae
done
clear
C)
Human Immunodeficiency Virus
done
clear
D)
Hepatitis - B virus
done
clear
View Answer play_arrow
question_answer 170) A phenomenon where the third base of r RNA at its 5 end can pair with a non - complementary base of mRNA is called
A)
universality
done
clear
B)
colinearity
done
clear
C)
degenerency
done
clear
D)
wobbling
done
clear
View Answer play_arrow
question_answer 171)
Given below are two statements A and B. Choose the correct answer related to the statements. Statement A: Ammo acids are amphoteric in their function. Statement B: All ammo acids are necessary for our body.
A)
Statement A is correct. Statement B is wrong
done
clear
B)
Both the Statements A and B are correct
done
clear
C)
Statement A is wrong. Statement B is correct
done
clear
D)
Both the Statements A and B are wrong
done
clear
View Answer play_arrow
question_answer 172) Plants like Aegle mormelos, Ocimum sanctum and Ficus religeosa are a group of plants designated as
A)
medicinal plant species
done
clear
B)
lesser known food plants
done
clear
C)
traditional food crops
done
clear
D)
sacred species of plants
done
clear
View Answer play_arrow
question_answer 173)
In the diagram given by the side, different parts are indicated by alphabets. Choose the answer in which these alphabets correctly match with the parts they indicate.
A)
A = Rostellum B = Hooks C = Sucker D = Proglottids
done
clear
B)
A = Suctorial mouth, B = Hooks, C = Sucker, D = Segments
done
clear
C)
A = Mouth, B = Tentacles, C = Sucker, D = Segments
done
clear
D)
A = Sucker, B = Hairs, C = Ring, D = Proglottids
done
clear
View Answer play_arrow
question_answer 174) In a vascular bundle, if xylem vessels develop in a centripetal fashion, the xylem is likely to be
A)
mesarch
done
clear
B)
centrarch
done
clear
C)
endarch
done
clear
D)
exarch
done
clear
View Answer play_arrow
question_answer 175) Which of the following plant material is an efficient water imbibant?
A)
Lignin
done
clear
B)
Pectin
done
clear
C)
Agar
done
clear
D)
Cellulose
done
clear
View Answer play_arrow
question_answer 176) Identify the plant parts whose transverse section show a clear and prominent pith
A)
dicot stem and monocot stem
done
clear
B)
dicot stem and monocot root
done
clear
C)
dicot root and monocot root
done
clear
D)
dicot stem and dicot root
done
clear
View Answer play_arrow
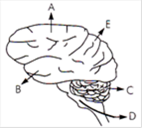
question_answer 177)
In the diagram of the lateral view of the human brain, parts are indicated by alphabets. Choose the answer in which these alphabets have been correctly matched with the part which they indicate.
A)
A = Temporal lobe B = Parietal lobe C = Cerebellum D = Medulla oblongata E = Frontal lobe
done
clear
B)
A = Frontal lobe B = Temporal lobe C = Cerebrum D = Medulla oblongata E = Occipetal lobe
done
clear
C)
A = Temporal lobe B = Parietal lobe C = Cerebrum D = Medulla oblongata E = Frontal lobe
done
clear
D)
A = Frontal lobe B = Temporal lobe C = Cerebellum D = Medulla oblongata E = Perietal lobe
done
clear
View Answer play_arrow
question_answer 178) Of all the environmental factors which is the most influential in determining the rate of transpiration?
A)
Light
done
clear
B)
Water
done
clear
C)
Relative humidity of atmosphere
done
clear
D)
Temperature
done
clear
View Answer play_arrow
question_answer 179) Curved portion of the Henles loop of the nephrons are lined by
A)
squamous epithelium
done
clear
B)
columnar epithelium
done
clear
C)
ciliated epithelium
done
clear
D)
cuboidal epithelium
done
clear
View Answer play_arrow
question_answer 180) In succulent plants like Opuntia, the RQ value will be
A)
less than 1
done
clear
B)
more than 1
done
clear
C)
infinity
done
clear
D)
zero
done
clear
View Answer play_arrow