A) Basicity of \[{{H}_{3}}P{{O}_{4}}\] and \[{{H}_{3}}P{{O}_{3}}\] is 3 and 3 respectively
B) Acidity of \[{{H}_{3}}P{{O}_{4}}\] and \[{{H}_{3}}P{{O}_{3}}\] is 3 and 3 respectively
C) Acidity of \[{{H}_{3}}P{{O}_{4}}\] and \[{{H}_{3}}P{{O}_{3}}\] is 3 and 2 respectively
D) Basicity of \[{{H}_{3}}P{{O}_{4}}\] and \[{{H}_{3}}P{{O}_{3}}\] is 3 and 2 respectively
Correct Answer: D
Solution :
Orthophosphoric acid \[({{H}_{3}}P{{O}_{4}})\]is a tribasic acid because it has three replaceable hydrogen atoms. Hence, the basicity of \[{{H}_{3}}P{{O}_{4}}\] is 3. Its structure is as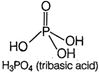 While phosphorus acid \[({{H}_{3}}P{{O}_{4}})\] is a dibasic acid because it has two replaceable hydrogen atom. Hence, the basicity of \[{{H}_{3}}P{{O}_{4}}\] is 2. Its structure is as
While phosphorus acid \[({{H}_{3}}P{{O}_{4}})\] is a dibasic acid because it has two replaceable hydrogen atom. Hence, the basicity of \[{{H}_{3}}P{{O}_{4}}\] is 2. Its structure is as 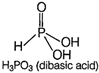
You need to login to perform this action.
You will be redirected in
3 sec
