A) \[\frac{x}{a}\]
B) \[\frac{a}{x}\]
C) \[\frac{x}{a\,(a-x)}\]
D) \[\frac{x}{{{a}^{2}}}\]
Correct Answer: C
Solution :
\[A+B\xrightarrow{{}}\]Product Initially a a 0 After time t \[(a-x)\] \[(a-x)\] \[x\] The rate constant, k is given by \[k=\frac{1}{t}\times \frac{x}{a\,(a-x)}\]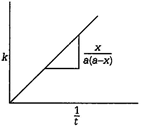
You need to login to perform this action.
You will be redirected in
3 sec
