A) The B atoms in it are \[s{{p}^{3}}\] hybridized
B) It contains two 3-centre-2-electron bonds
C) All B-H bond lengths in it are equal due to resonance
D) The molecule is non-planar
E) The molecule contains 12 valence electrons
Correct Answer: C
Solution :
The structure of \[{{B}_{2}}{{H}_{6}}\](diborane) is as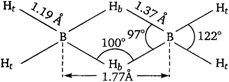 The bond length between \[B-{{H}_{t}}\] and \[B-{{H}_{b}}\] are 1.19 \[\overset{\text{o}}{\mathop{\text{A}}}\,\] and 1.37 \[\overset{\text{o}}{\mathop{\text{A}}}\,\] respectively. \[B-{{H}_{b}}\] is longer than \[B-{{H}_{t}}\]due to electron deficiency. Therefore, statement about diborane is not true.
The bond length between \[B-{{H}_{t}}\] and \[B-{{H}_{b}}\] are 1.19 \[\overset{\text{o}}{\mathop{\text{A}}}\,\] and 1.37 \[\overset{\text{o}}{\mathop{\text{A}}}\,\] respectively. \[B-{{H}_{b}}\] is longer than \[B-{{H}_{t}}\]due to electron deficiency. Therefore, statement about diborane is not true.
You need to login to perform this action.
You will be redirected in
3 sec
