A) E2 mechanism
B) E1 mechanism
C) due to rearrangement of carbocation by E1 mechanism
D) E1 CB mechanism
E) Hermann elimination
Correct Answer: C
Solution :
Neopentyl bromide undergoes dehydro-halogenation to give alkene even though it has no \[\beta \text{-}\]hydrogen atom. This is due to rearrangement of carbocation by \[{{E}_{1}}\]mechanism. \[C{{H}_{3}}-\underset{C{{H}_{3}}}{\overset{C{{H}_{3}}}{\mathop{\underset{\mathbf{|}}{\overset{\mathbf{|}}{\mathop{C}}}\,}}}\,-C{{H}_{2}}Br\xrightarrow[-B{{r}^{-}}]{}\]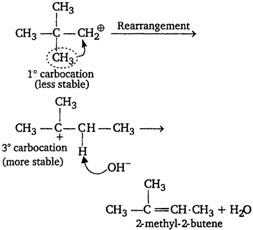
You need to login to perform this action.
You will be redirected in
3 sec
