A) \[NaCl\]
B) \[NaF\]
C) \[NaOH\]
D) \[NaN{{O}_{3}}\]
E) \[NaBr\]
Correct Answer: E
Solution :
Concentrated sulphuric acid, being a strong acid, oxidises bromides and iodides but not chlorides and fluorides since, the latter are more electronegative. Hence, it, can be reduced by only\[NaBr\]among the given options. \[\overset{+\,6}{\mathop{{{H}_{2}}S{{O}_{4}}}}\,+\overset{-1}{\mathop{NaBr}}\,\xrightarrow{{}}\overset{+\,6}{\mathop{NaHS{{O}_{4}}}}\,+\overset{-1}{\mathop{HBr}}\,\]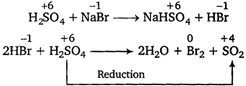
You need to login to perform this action.
You will be redirected in
3 sec
