question_answer 1) The gas in a vessel is subjected to a pressure of 20 atm at a temperature \[27{}^\circ C\]. The pressure of gas in a vessel after one-half of the gas is released from the vessel and the temperature of the remainder is raised by \[50{}^\circ C\] is
A)
8.5 atm
done
clear
B)
10.8 atm
done
clear
C)
11.7 atm
done
clear
D)
17 atm
done
clear
View Answer play_arrow
question_answer 2) A certain radioactive substance has a half-life of 5 years. Thus, for a nucleus in a sample of the elements, the probability of decay in 10 years is
A)
50%
done
clear
B)
75%
done
clear
C)
100%
done
clear
D)
60%
done
clear
View Answer play_arrow
question_answer 3) A reversible engine working between the temperature limits of 600 K and 1200 K receives 50 kJ of heat. The work done by the engine will be
A)
50 kJ
done
clear
B)
100 kJ
done
clear
C)
25 kJ
done
clear
D)
-25 kJ
done
clear
View Answer play_arrow
question_answer 4) A particle moving, with a velocity equal to 0.4 m/s is subjected to an acceleration of 0.15 \[m\text{/}{{s}^{2}}\] for 2 s in a direction at right angles to its direction of motion. The resultant velocity is
A)
0.7 m/s
done
clear
B)
0.5 m/s
done
clear
C)
0.1 m/s
done
clear
D)
between 0.7 and 0.1 m/s
done
clear
View Answer play_arrow
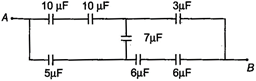
question_answer 5)
In the figure, the equivalence capacitance between A and B is
A)
3.75\[\mu F\]
done
clear
B)
5.25\[\mu F\]
done
clear
C)
6.5\[\mu F\]
done
clear
D)
10.5\[\mu F\]
done
clear
View Answer play_arrow
question_answer 6) A scooter of mass 120 kg is moving with a uniform velocity of 108 km/h, the force required to stop the vehicle in 10 s is
A)
360 N
done
clear
B)
720 N
done
clear
C)
180 N
done
clear
D)
120\[\times \]108 N
done
clear
View Answer play_arrow
question_answer 7) In a shunted ammeter, only 10% of current passes through the galvanometer of resistance G. The resistance of the shunt is
A)
\[9\,G\]
done
clear
B)
\[10\,G\]
done
clear
C)
\[\frac{G}{9}\]
done
clear
D)
\[\frac{G}{10}\]
done
clear
View Answer play_arrow
question_answer 8) Two bodies of masses 10 kg and 100 kg are separated by a distance of 2 m. The gravitational potential at the mid-point on the line joining the bodies is
A)
\[7.3\times {{10}^{-7}}J\text{/}kg\]
done
clear
B)
\[7.3\times {{10}^{-8}}J\text{/}kg\]
done
clear
C)
\[7.3\times {{10}^{-9}}J\text{/}kg\]
done
clear
D)
\[7.3\times {{10}^{-6}}J\text{/}kg\]
done
clear
View Answer play_arrow
question_answer 9) The decay constant A. of a radioactive sample is the probability of decay of an atom in unit time. Then,
A)
\[\lambda \] decreases as the atom becomes older
done
clear
B)
\[\lambda \] is independent of the age of atoms
done
clear
C)
\[\lambda \] increases as the age of atom
done
clear
D)
behaviour of \[\lambda \] with time depends on the nature of the activity
done
clear
View Answer play_arrow
question_answer 10) The average power dissipation is pure inductance is
A)
\[\frac{1}{2}L{{I}^{2}}\]
done
clear
B)
\[2\,L{{I}^{2}}\]
done
clear
C)
\[\frac{1}{4}\,L{{I}^{2}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 11) \[n\] identical mercury droplets charged to the same potential V coalesce to form a single bigger drop. The potential of new drop will be
A)
\[\frac{V}{n}\]
done
clear
B)
\[nV\]
done
clear
C)
\[n{{V}^{2}}\]
done
clear
D)
\[{{n}^{2/3}}V\]
done
clear
View Answer play_arrow
question_answer 12) For protecting sensitive equipment from external magnetic field, it should be
A)
wrapped with insulation around it when passing current through it
done
clear
B)
placed inside an iron core
done
clear
C)
surrounded with we shear
done
clear
D)
placed inside aluminium core
done
clear
View Answer play_arrow
question_answer 13) The potential energy of a charged parallel plate capacitor is\[{{U}_{0}}.\] If a slab of dielectric constant \[K\] is increased between the plates, then the new potential energy will be
A)
\[\frac{{{U}_{0}}}{K}\]
done
clear
B)
\[{{U}_{0}}{{K}^{2}}\]
done
clear
C)
\[\frac{{{U}_{0}}}{{{K}^{2}}}\]
done
clear
D)
\[U_{0}^{2}\]
done
clear
View Answer play_arrow
question_answer 14) Dimension of self-inductance are
A)
\[[ML{{T}^{-2}}{{A}^{-3}}]\]
done
clear
B)
\[[M{{L}^{-2}}{{T}^{-2}}{{A}^{-1}}]\]
done
clear
C)
\[[M{{L}^{2}}{{T}^{-2}}{{A}^{-2}}]\]
done
clear
D)
\[[M{{L}^{-2}}{{T}^{-2}}{{A}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 15) The increase in length of a wire on stretching is 0.025%. If its poisons ratio is 0.4, then the percentage decrease in the diameter is
A)
0.01
done
clear
B)
0.02
done
clear
C)
0.03
done
clear
D)
0.04
done
clear
View Answer play_arrow
question_answer 16) The maximum height reached by a projectile is 4m. The horizontal range is 12m. Velocity of projection in \[m{{s}^{-1}},\]is (g = acceleration due to gravity)
A)
\[5\sqrt{\frac{g}{2}}\]
done
clear
B)
\[3\frac{g}{\sqrt{2}}\]
done
clear
C)
\[\frac{1}{3}\frac{g}{\sqrt{2}}\]
done
clear
D)
\[\frac{1}{5}\sqrt{\frac{g}{2}}\]
done
clear
View Answer play_arrow
question_answer 17) Four particles, each of mass 1 kg, are placed at the corners of a square of side 1m in the X-Y plane. If the point of intersection of the diagonals of the square, is taken as the origin the coordinates of the centre of mass are
A)
(1, 1)
done
clear
B)
(-1, 1)
done
clear
C)
(1, -1)
done
clear
D)
(0, 0)
done
clear
View Answer play_arrow
question_answer 18) Two rain drops reach of the earth with their terminal velocities in the ratio 4 : 9. The ratio of their radii is,
A)
4 : 9
done
clear
B)
2 : 3
done
clear
C)
3 : 2
done
clear
D)
9 : 4
done
clear
View Answer play_arrow
question_answer 19) Two bodies of same shape, same size and same radiating power have emissivitys 0.2 and 0.8. The ratio of their temperatures is
A)
\[\sqrt{3}:1\]
done
clear
B)
\[\sqrt{2}:1\]
done
clear
C)
\[1:\sqrt{5}\]
done
clear
D)
\[1:\sqrt{8}\]
done
clear
View Answer play_arrow
question_answer 20) Water from a tap emerges vertically downwards with initial velocity\[4\,m{{s}^{-1}}\]. The cross-sectional area of the tap is A. The flow is steady and pressure is constant throughout the stream of water. The distance h vertically below the tap, where the cross-sectional area of the stream becomes \[\left( \frac{2}{3} \right)\]A, is (g = 10\[m\text{/}{{s}^{2}}\])
A)
0.5 m
done
clear
B)
1 m
done
clear
C)
1.5 m
done
clear
D)
2.2 m
done
clear
View Answer play_arrow
question_answer 21) A short magnetic needle is pivoted in a uniform magnetic field of induction 1 T. Now, simultaneously another magnetic field of induction \[\sqrt{3}\]T is applied at right angles to the first field, the needle deflects through an angle 8 whose value is
A)
\[30{}^\circ \]
done
clear
B)
\[45{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
\[60{}^\circ \]
done
clear
View Answer play_arrow
question_answer 22) The potential difference between two parallel plates is\[{{10}^{4}}V\]. If the plates are separated by 0.5 cm, the force on an electron between the plates is
A)
\[32\times {{10}^{-13}}N\]
done
clear
B)
\[0.32\times {{10}^{-13}}N\]
done
clear
C)
\[0.032\times {{10}^{-13}}N\]
done
clear
D)
\[3.2\times {{10}^{-13}}N\]
done
clear
View Answer play_arrow
question_answer 23) The diameter of objective of a telescope is 1 m. Its resolving limit for the light of wavelength 4538\[\overset{\text{o}}{\mathop{\text{A}}}\,\], will be
A)
\[5.54\times {{10}^{-7}}\text{rad}\]
done
clear
B)
\[2.54\times {{10}^{-4}}\text{rad}\]
done
clear
C)
\[6.54\times {{10}^{-7}}\text{rad}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 24) A ball is falling freely from a height. When, it reaches 10 m height from the ground its velocity is \[{{v}_{0}}\]. It collides with the ground and loses 50% of its energy and rises back to height of 10 m. Then, the velocity\[{{v}_{0}}\]is
A)
7 m/s
done
clear
B)
10 m/s
done
clear
C)
14 m/s
done
clear
D)
16 m/s
done
clear
View Answer play_arrow
question_answer 25) A fly-wheel of mass 25 kg has a radius of 0.2m It is making 240 rpm. What is the torque necessary to bring to rest in 20 s?
A)
\[2\pi \,N\text{-}m\]
done
clear
B)
\[0.4\pi \,N\text{-}m\]
done
clear
C)
\[\frac{2}{\pi }\,N\text{-}m\]
done
clear
D)
\[4\pi \,N\text{-}m\]
done
clear
View Answer play_arrow
question_answer 26) Given the following sequence of reactions,\[C{{H}_{3}}C{{H}_{2}}I\xrightarrow{NaCN}A\xrightarrow[Partial\,\,hydrolysis]{O{{H}^{-}}}B\]\[\xrightarrow[{}]{B{{r}_{2}}/NaOH}C\] The major product C is
A)
\[C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}\underset{O}{\mathop{\underset{\mathbf{|}\,\,\mathbf{|}}{\mathop{C}}\,}}\,-NHBr\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}-COON{{H}_{4}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}-\underset{O}{\mathop{\underset{|\,\,|}{\mathop{C}}\,}}\,-NB{{r}_{2}}\]
done
clear
View Answer play_arrow
question_answer 27) Nylon-66 is obtained by the condensation polymerisation of
A)
adipic acid and hexamethylene diamine
done
clear
B)
phenol and formaldehyde
done
clear
C)
terephthalic acid and ethylene glycol
done
clear
D)
sebacic acid and hexamethylene
done
clear
View Answer play_arrow
question_answer 28) The final product in the following sequence of reaction is\[CH\equiv CH\xrightarrow[{}]{NaN{{H}_{2}}}A\xrightarrow[{}]{C{{H}_{3}}Br}B\]
A)
\[C{{H}_{2}}=CH-CH=C{{H}_{2}}\]
done
clear
B)
\[HC\equiv C-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{2}}=CH-C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 29)
The IUPAC name of following compound is
A)
N, N-dimethyl- 3-methyl pentan-3-amine
done
clear
B)
3-N, N-dimethyl, 3-methyl pentanamine
done
clear
C)
3-methyl-3-N, N-dimethyl pentane
done
clear
D)
3-methyl-3-N, N-dimethyl butane
done
clear
View Answer play_arrow
question_answer 30) Which of the following compounds is known as the antisterility factor?
A)
\[\alpha \text{-}\]tocopherol
done
clear
B)
Retinol
done
clear
C)
Calciferol
done
clear
D)
Pyridoxine
done
clear
View Answer play_arrow
question_answer 31) A 400 mg iron capsule contains 100 mg of ferrous fumarate, \[{{(CHCOO)}_{2}}Fe\,.\] The percentage of iron present in it is approximately
A)
33%
done
clear
B)
25%
done
clear
C)
14%
done
clear
D)
8%
done
clear
View Answer play_arrow
question_answer 32) Which of the following has maximum dipole moment?
A)
\[NC{{l}_{3}}\]
done
clear
B)
\[NBr\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[N{{I}_{3}}\]
done
clear
View Answer play_arrow
question_answer 33)
Gases X, Y, Z, P and Q have the van der Waals constants a and b (in CGS units) as shown below. X Y Z P Q A 6 6 20 0.05 30 B 0.025 0.15 0.1 0.02 0.2
The gas with the higher critical temperature is
A)
P
done
clear
B)
Q
done
clear
C)
Y
done
clear
D)
X
done
clear
View Answer play_arrow
question_answer 34) The incorrect statement among the following is
A)
the first ionisation potential of Al is less than the first ionisation potential of Mg
done
clear
B)
the second ionisation potential of Mg is greater than the second ionisation potential of Na
done
clear
C)
the first ionisation potential of Na is less than the first ionisation potential of Mg
done
clear
D)
the third ionisation potential of Mg is greater than that of Al
done
clear
View Answer play_arrow
question_answer 35)
When \[NaN{{O}_{3}}\] is heated in a closed vessel, \[{{O}_{2}}\]is liberated and \[NaN{{O}_{2}}\]is left behind. At equilibrium, I. addition of\[NaN{{O}_{3}}\]favours forward reaction II. addition of \[NaN{{O}_{2}}\]favours backward reaction III. increasing pressure favours reverse reaction IV. increasing temperature favours forward reaction Correct options are
A)
I, II and III
done
clear
B)
II, III and IV
done
clear
C)
I, III and IV
done
clear
D)
I, II, III and IV
done
clear
View Answer play_arrow
question_answer 36) If 900 J/g of heat is exchanged at boiling point of water then increase in entropy is
A)
43.4 J/mol
done
clear
B)
87.2 J/mol
done
clear
C)
900 J/mol
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 37) A solid is a compound of group 1 element and it gives a bright red colour in the flame test. The solid is
A)
LiBr
done
clear
B)
CsCI
done
clear
C)
KCI
done
clear
D)
NaCI
done
clear
View Answer play_arrow
question_answer 38) Mixture of sugar and common salt is separate by crystallisation by dissolving in
A)
\[{{H}_{2}}O\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[{{C}_{6}}{{H}_{6}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 39) Identify the product C in the following series of reactions,\[\text{Glucose}\xrightarrow{HCN}A\xrightarrow{{{H}_{2}}O}B\xrightarrow{HI}C\]
A)
heptanoic acid
done
clear
B)
hexanoic acid
done
clear
C)
\[\alpha \text{-}\]methyl caproic acid
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 40)
A)
It has analgesic as well as antipyretic properties
done
clear
B)
It helps to prevent heart attack
done
clear
C)
It has anti-blood clotting action
done
clear
D)
It suppresses the gastric anomalies
done
clear
View Answer play_arrow
question_answer 41) Optical isomerism is shown by octahedral complexes
A)
having all monodentate ligands
done
clear
B)
having all the three bidentate ligands
done
clear
C)
having two trans bidentate ligands
done
clear
D)
having two trans monodentate ligands
done
clear
View Answer play_arrow
question_answer 42)
A)
\[C{{H}_{3}}-\overset{C{{H}_{3}}}{\mathop{\overset{\mathbf{|}}{\mathop{CH}}\,}}\,-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-\underset{C{{H}_{3}}}{\overset{C{{H}_{3}}}{\mathop{\underset{\mathbf{|}}{\overset{\mathbf{|}}{\mathop{C}}}\,}}}\,-C{{H}_{3}}\]
done
clear
C)
done
clear
D)
\[C{{H}_{3}}-\overset{C{{H}_{3}}}{\mathop{\overset{\mathbf{|}}{\mathop{CH}}\,}}\,-C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 43) The Brownian movement is due to
A)
enthalpy change during the formation of colloids
done
clear
B)
attractive forces between the colloidal particles and the molecules of dispersion medium
done
clear
C)
the impact of molecules of the dispersion medium on the colloidal particles
done
clear
D)
the movement of positively charged colloidal particle to negatively charged particle
done
clear
View Answer play_arrow
question_answer 44) The standard reduction potential,\[E{}^\circ \]for the half-reactions are as \[Zn\,\,\,\,Z{{n}^{2+}}+2{{e}^{-}};\] \[E{}^\circ =+\,0.76\,V\] \[Fe\,\,\,\,F{{e}^{2+}}+2{{e}^{-}};\]\[E{}^\circ =+\,0.41\,V\] The \[E_{cell}^{\text{o}}\] for the cell formed by these two electrodes is
A)
- 0.35 V
done
clear
B)
- 1.17 V
done
clear
C)
+ 0.35 V
done
clear
D)
+ 1.17 V
done
clear
View Answer play_arrow
question_answer 45) The relationship between osmotic pressure at 273 K when 10 g glucose \[({{p}_{1}}),\] 10 g urea \[({{p}_{2}})\]and 10 g sucrose \[({{p}_{3}})\] are dissolved in 250 mL of water is
A)
\[{{p}_{1}}>{{p}_{2}}>{{p}_{3}}\]
done
clear
B)
\[{{p}_{3}}>{{p}_{2}}>{{p}_{1}}\]
done
clear
C)
\[{{p}_{2}}>{{p}_{1}}>{{p}_{3}}\]
done
clear
D)
\[{{p}_{2}}>{{p}_{3}}>{{p}_{1}}\]
done
clear
View Answer play_arrow
A)
Only I
done
clear
B)
Only II
done
clear
C)
Only III
done
clear
D)
I and II
done
clear
View Answer play_arrow
question_answer 47) Which one is correct statement?
A)
Basicity of \[{{H}_{3}}P{{O}_{4}}\] and \[{{H}_{3}}P{{O}_{3}}\] is 3 and 3 respectively
done
clear
B)
Acidity of \[{{H}_{3}}P{{O}_{4}}\] and \[{{H}_{3}}P{{O}_{3}}\] is 3 and 3 respectively
done
clear
C)
Acidity of \[{{H}_{3}}P{{O}_{4}}\] and \[{{H}_{3}}P{{O}_{3}}\] is 3 and 2 respectively
done
clear
D)
Basicity of \[{{H}_{3}}P{{O}_{4}}\] and \[{{H}_{3}}P{{O}_{3}}\] is 3 and 2 respectively
done
clear
View Answer play_arrow
question_answer 48) The volume strength of 1.5 N \[{{H}_{2}}{{O}_{2}}\]solution is
A)
16.8 L
done
clear
B)
8.4 L
done
clear
C)
4.2 L
done
clear
D)
5.2 L
done
clear
View Answer play_arrow
question_answer 49) The oxidation number of oxygen in \[O{{F}_{2}}\]is
A)
+2
done
clear
B)
-2
done
clear
C)
+1
done
clear
D)
-1
done
clear
View Answer play_arrow
question_answer 50) If uncertainties in the measurement of position and momentum of an electron are equal, the uncertainty in the measurement of velocity is
A)
\[8.0\times {{10}^{12}}m{{s}^{-1}}\]
done
clear
B)
\[4.2\times {{10}^{10}}m{{s}^{-1}}\]
done
clear
C)
\[8.5\times {{10}^{10}}m{{s}^{-1}}\]
done
clear
D)
\[6.2\times {{10}^{10}}m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 51) The largest RBCs among vertebrates are found in
A)
amphibians
done
clear
B)
reptiles
done
clear
C)
mammals
done
clear
D)
aves
done
clear
View Answer play_arrow
question_answer 52) Which of the following algae is used as a source of proteins?
A)
Porphyra tenera
done
clear
B)
Chlorella
done
clear
C)
Spirulina
done
clear
D)
Chara
done
clear
View Answer play_arrow
question_answer 53) The Imperial Forest Research Institute (IFRI) established in 1906 changed its name to
A)
IARI
done
clear
B)
FRI
done
clear
C)
CDRI
done
clear
D)
CSIR
done
clear
View Answer play_arrow
question_answer 54) Choose the genetically engineered variety of organisms created to deal with the problem of oil leakage
A)
Agrobacterium rhizogenes
done
clear
B)
Agrobacterium tumefaciens
done
clear
C)
Escherichia coli
done
clear
D)
Pseudomonas putida
done
clear
View Answer play_arrow
question_answer 55) Klinefelters syndrome is a chromosomal abnormality in humans. It is linked to
A)
21 chromosome
done
clear
B)
44 autosomes + XO
done
clear
C)
44 autosomes + XXY
done
clear
D)
1st chromosome
done
clear
View Answer play_arrow
question_answer 56) Pollens are preserved for long time in fossils due to presence of
A)
pectocellulose
done
clear
B)
cuticle
done
clear
C)
sporopollenin
done
clear
D)
germ pores
done
clear
View Answer play_arrow
question_answer 57) Latimeria (coelocanth) is a
A)
living fossil
done
clear
B)
connecting link between fishes and amphibians
done
clear
C)
bony fish,
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 58)
Identify the incorrect statement about DNA. I. Base composition of DNA varies from one species to another. II. Z-DNA is a right handed helix. III. Prokaryotic genomic DNA and many viral DNAs are circular molecules. IV. Hershey and Chase experiments observed the transformation in mice.
A)
I and II
done
clear
B)
II and III
done
clear
C)
II and IV
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 59) The crunchy and granular feeling when we chew pear fruit is due to
A)
parenchyma
done
clear
B)
collenchyma
done
clear
C)
chlorenchyma
done
clear
D)
sclerenchyma
done
clear
View Answer play_arrow
question_answer 60) A bone that lies at the base of the tongue and is not articulated with rest of the skeleton is
A)
maxilla
done
clear
B)
hyoid
done
clear
C)
coccyx
done
clear
D)
innominate
done
clear
View Answer play_arrow
question_answer 61)
Match the following columns. Column-I (Disease) Column-II (Vector) A. Yellow fever 1. Tse-tse fly B. Sleeping sickness 2. Sandfly C. Filariasis 3. Aedes D. Kala-azar 4. Culex
Codes
A)
A-1 B-2 C-3 D-4
done
clear
B)
A-3 B-1 C-4 D-2
done
clear
C)
A-2 B-4 C-1 D-3
done
clear
D)
A-4 B-2 C-3 D-1
done
clear
View Answer play_arrow
question_answer 62) Dark reaction in photosynthesis is called so because
A)
it does not depend on light energy
done
clear
B)
it can occur in dark also
done
clear
C)
it cannot occur during day light
done
clear
D)
it occurs more rapidly at night
done
clear
View Answer play_arrow
question_answer 63) A pigment called leghaemoglobin is present in the root nodules of legumes. Its function is
A)
convert atmospheric \[{{N}_{2}}\] to\[N{{H}_{3}}\]
done
clear
B)
transport \[{{O}_{2}}\]for activity of nitrogenase
done
clear
C)
convert ammonia to nitrate
done
clear
D)
protect nitrogenase from oxygen
done
clear
View Answer play_arrow
question_answer 64) The theory where ratio between number of X chromosomes and number of complete set of autosomes determine sex is
A)
chromosome theory of sex determination
done
clear
B)
genie balance theory of sex determination
done
clear
C)
environmental sex determination theory
done
clear
D)
hormonal balance theory of sex determination
done
clear
View Answer play_arrow
question_answer 65) Which of the following is correctly matched?
A)
Glycolysis - Inner mitochondrial membrane
done
clear
B)
Krebs cycle - Mitochondrial matrix
done
clear
C)
Electron transport system - Cytosol
done
clear
D)
Fermentation - Mitochondrial matrix
done
clear
View Answer play_arrow
question_answer 66) In grass-deer-tiger food chain, grass biomass is 1 tonne. The tiger biomass shall be
A)
100 kg
done
clear
B)
200 kg
done
clear
C)
10 kg
done
clear
D)
1 kg
done
clear
View Answer play_arrow
question_answer 67) The venom of honeybees is used in the treatment of
A)
arthritis
done
clear
B)
diphtheria
done
clear
C)
pertussis
done
clear
D)
diabetes
done
clear
View Answer play_arrow
question_answer 68) A plant cell with 0.5% concentration of salt in its cell sap is placed in a solution with 5% salt concentration. The cell will
A)
swell due to exosmosis
done
clear
B)
show no change in shape
done
clear
C)
shrink due to endosmosis
done
clear
D)
shrink due to exosmosis
done
clear
View Answer play_arrow
question_answer 69) What is correct about test-tube baby?
A)
Fertilisation inside female genital tract and growth in test-tube:
done
clear
B)
Fertilisation outside and gestation inside womb of mother
done
clear
C)
Premature born baby reared in incubator
done
clear
D)
Both fertilisation and development outside the female genital tract
done
clear
View Answer play_arrow
question_answer 70)
Match the items in Column I with those of Column II. Column I Column II A. Polymerase chain reaction 1. Banting and Best B. Insulin 2. Chemical scalpel C. Eli Lilly 3. Humulin D. Restriction enzymes 4. Kornberg 5. Kary Mullis
A)
A-4 B-5 C-3 D-2
done
clear
B)
A-1 B-3 C-2 D-4
done
clear
C)
A-5 B-1 C-3 D-2
done
clear
D)
A-1 B-4 C-3 D-5
done
clear
View Answer play_arrow
question_answer 71) A fleshy false fruit which develops from fleshy thalamus is
A)
drupe
done
clear
B)
berry
done
clear
C)
pome
done
clear
D)
pepo
done
clear
View Answer play_arrow
question_answer 72) Statement I Jelly fish and dog fish belong to the same class. Statement II Both of them are aquatic organisms and possess a hard exoskeleton. Choose the correct option
A)
Statement I is correct but-statement II is incorrect
done
clear
B)
Statement II is correct but statement I is incorrect
done
clear
C)
Both statements are correct
done
clear
D)
Both statements are incorrect
done
clear
View Answer play_arrow
question_answer 73) Competitive inhibition could be reversed by increasing the concentration of
A)
coenzyme
done
clear
B)
substrate
done
clear
C)
product
done
clear
D)
enzyme
done
clear
View Answer play_arrow
question_answer 74) Which of the following pair is correctly matched?
A)
Cardiac sphincter-Between oesophagus and anterior stomach
done
clear
B)
Pyloric sphincter-Between small intestine and bowl
done
clear
C)
Sphincter of oddi - Terminal part of alimentary canal
done
clear
D)
lleocaecal sphincter-Between duodenum and posterior stomach
done
clear
View Answer play_arrow
question_answer 75) A man suffering from diabetes mellitus drinks water more frequently as he has to
A)
eliminate extra salt from blood
done
clear
B)
eliminate extra glucose from blood
done
clear
C)
eliminate extra insulin from blood
done
clear
D)
eliminate extra water from blood
done
clear
View Answer play_arrow
question_answer 76) Which of the following schemes was launched to promote basic education in India and attract children in school going age to attend the classes?
A)
Pulse Polio Abhiyan
done
clear
B)
Operation Flood
done
clear
C)
Mid-Day Meal Scheme
done
clear
D)
Operation Black Board
done
clear
View Answer play_arrow
question_answer 77) Yen is the currency of
A)
Japan
done
clear
B)
Taiwan
done
clear
C)
North Korea
done
clear
D)
South Korea
done
clear
View Answer play_arrow
question_answer 78) The bank rate is the rate of interest at which the Reserve Bank of India provides loans to the
A)
scheduled commercial banks
done
clear
B)
public sector
done
clear
C)
corporate sector
done
clear
D)
foreign institutional investors
done
clear
View Answer play_arrow
question_answer 79) Which of the following is not one of the Fundamental Rights?
A)
Right to freedom of religion
done
clear
B)
Right to freedom of thought and expression
done
clear
C)
Right to equality
done
clear
D)
Right to equal pay for equal work for man as well as worqan
done
clear
View Answer play_arrow
question_answer 80) Panchayati Raj System in India is laid down under
A)
Fundamental Rights
done
clear
B)
Fundamental Duties
done
clear
C)
Directive Principles of State Policy
done
clear
D)
Election Commission Act
done
clear
View Answer play_arrow
question_answer 81) What is the tenure of the Prime Minister of India?
A)
Conterminous with the tenure of the Lok Sabha
done
clear
B)
Conterminous with the tenure of the President
done
clear
C)
As long as he enjoys the support of a majority in the Lok Sabha
done
clear
D)
Five years
done
clear
View Answer play_arrow
question_answer 82) The words Satyameva Jayate in the state Emblem of India have been adopted from which one of the following?
A)
Mundak upanishad
done
clear
B)
Brahma Upanishad
done
clear
C)
Mudgala upanishad
done
clear
D)
Maitreyi upnishad
done
clear
E)
Aryabhatta
done
clear
View Answer play_arrow
question_answer 83) Ramayan the Tamil version of the great epic Ramayana was made by
A)
Kamban
done
clear
B)
Awaiyar
done
clear
C)
llango adigal
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 84) Which of the following is true about sedimentary rock?
A)
They are rocks whose structure is contingent on heat and pressure
done
clear
B)
The rocks are crystalline
done
clear
C)
The rocks have been deposited in layers
done
clear
D)
The rocks cannot be formed under water
done
clear
View Answer play_arrow
question_answer 85) Which of the following pair is not correctly matched?
A)
Himalayas -tertiary fold mountain
done
clear
B)
Deccan Trap-volcanic cone eruption
done
clear
C)
Western Ghat -palaeozoic fold mountains
done
clear
D)
Aravalli-pre-combrian rlict mountain
done
clear
View Answer play_arrow
question_answer 86) Which one of the following is a fungus?
A)
Agaricus
done
clear
B)
Funaria
done
clear
C)
Rhizobium
done
clear
D)
Spirogyra
done
clear
View Answer play_arrow
question_answer 87) Study of nose and olfactory organs is called
A)
radiology
done
clear
B)
rhinology
done
clear
C)
radiography
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 88) The temperature of a Gas is measured with a
A)
platinum resistance thermometer
done
clear
B)
pyrometer
done
clear
C)
gas thermometer
done
clear
D)
vapour pressure thermometer
done
clear
View Answer play_arrow
question_answer 89) A computer portable and easy to carry by travellers is
A)
super computer
done
clear
B)
laptop
done
clear
C)
mini computer
done
clear
D)
file servers
done
clear
View Answer play_arrow
question_answer 90) Many individuals of the same species living together in a defined area form a/an
A)
community
done
clear
B)
genus
done
clear
C)
population
done
clear
D)
ecosystem
done
clear
View Answer play_arrow
question_answer 91) In the past decade, which of the following has not been a major cause of the increase in the worlds population?
A)
Longer life span
done
clear
B)
Lower infant mortality
done
clear
C)
Increase in birth rate
done
clear
D)
Improved sanitation
done
clear
View Answer play_arrow
question_answer 92) Rangaswami cup is associated with
A)
wrestling
done
clear
B)
football
done
clear
C)
hockey
done
clear
D)
golf
done
clear
View Answer play_arrow
question_answer 93) April 2 is observed as
A)
world day for war orphans
done
clear
B)
united nations day for South-South cooperation
done
clear
C)
world autism awareness day, recognized by the UN
done
clear
D)
international day for Biological Diversity, recognized by the UN
done
clear
View Answer play_arrow
question_answer 94) ISRO is the abbreviation for
A)
Indian Scientific Research Organization
done
clear
B)
Indian Space Research Organization
done
clear
C)
International Space Research Organization
done
clear
D)
International Scientific Research Organization
done
clear
View Answer play_arrow
question_answer 95) Saline Alt was an eminent
A)
Urdu poet
done
clear
B)
Ornithologist
done
clear
C)
Ghazal singer
done
clear
D)
None of these
done
clear
View Answer play_arrow
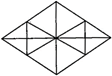
question_answer 96)
How many number of triangles in the following figure?
A)
16
done
clear
B)
22
done
clear
C)
28
done
clear
D)
32
done
clear
View Answer play_arrow
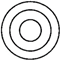
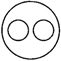
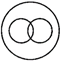
question_answer 97) Which of the following diagram best depicts the relationship among factory, machinery and product?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 98) The smallest number of 5 digits beginning with 3 and ending with 5 is
A)
30015
done
clear
B)
31005
done
clear
C)
30005
done
clear
D)
50003
done
clear
View Answer play_arrow
question_answer 99) Directions: Read each sentence to find out whether there is any grammatical error in it. The error, if any, will be in one part of the sentence. The number of that part is the answer. If there is no error, the answer is (d) i.e., No error (Ignore errors of punctuation, if any),
A)
Handsome is usually used
done
clear
B)
/ of men but beautiful is not
done
clear
C)
/ usually used to talk about mans appearance,
done
clear
D)
/ No error
done
clear
View Answer play_arrow
question_answer 100) Directions: Read each sentence to find out whether there is any grammatical error in it. The error, if any, will be in one part of the sentence. The number of that part is the answer. If there is no error, the answer is i.e., No error (Ignore errors of punctuation, if any),
A)
Payal kept her drum
done
clear
B)
/besides her
done
clear
C)
/ always and she played wisely.
done
clear
D)
/ No error
done
clear
View Answer play_arrow
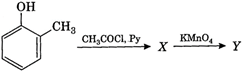 The final product Y is medicine. Which of the following is incorrect regarding V ?
The final product Y is medicine. Which of the following is incorrect regarding V ?