question_answer 1) If a current of 500 mA produces a deflection of \[30{}^\circ \] in a tangent galvanometer, then the current that produces a deflection of \[60{}^\circ \]is
A)
1.5 A
done
clear
B)
1 A
done
clear
C)
500 mA
done
clear
D)
866 mA
done
clear
E)
2A
done
clear
View Answer play_arrow
question_answer 2) A wire of length 50 cm moves with a velocity of 300 m/min, perpendicular to a magnetic field. If the emf induced in the wire is 2 V, the magnitude of the field in tesia is
A)
2
done
clear
B)
5
done
clear
C)
0.4
done
clear
D)
2.5
done
clear
E)
0.8
done
clear
View Answer play_arrow
question_answer 3) Whenever a magnet is moved either towards or away from a conducting coil, an emf is induced, the magnitude of which is independent of
A)
the strength of the magnetic field
done
clear
B)
the speed with which the magnet is moved
done
clear
C)
the number of turns is the coil
done
clear
D)
the resistance of the coil
done
clear
E)
the area of cross-section of the coil
done
clear
View Answer play_arrow
question_answer 4) An electromagnetic radiation has an energy of 13.2 keV. Then the radiation belongs to the region of
A)
visible light
done
clear
B)
ultraviolet
done
clear
C)
infrared
done
clear
D)
X-ray
done
clear
E)
microwave
done
clear
View Answer play_arrow
question_answer 5) The photosensitive surface is receiving light of wavelength 5000\[\overset{\text{o}}{\mathop{\text{A}}}\,\] at the rate of \[{{10}^{-8}}J\text{/}s.\] The number of photons received per second is
A)
\[2.5\times {{10}^{10}}\]
done
clear
B)
\[2.5\times {{10}^{11}}\]
done
clear
C)
\[2.5\times {{10}^{12}}\]
done
clear
D)
\[2.5\times {{10}^{9}}\]
done
clear
E)
\[2.5\times {{10}^{13}}\]
done
clear
View Answer play_arrow
question_answer 6) The nucleus\[{}_{6}{{C}_{12}}\]absorbs an energetic neutron and emits a beta particle\[(\beta )\]. The resulting nucleus is
A)
\[{}_{7}{{N}^{14}}\]
done
clear
B)
\[{}_{7}{{N}^{13}}\]
done
clear
C)
\[{}_{5}{{B}^{13}}\]
done
clear
D)
\[{}_{6}{{C}^{13}}\]
done
clear
E)
\[{}_{5}{{B}^{12}}\]
done
clear
View Answer play_arrow
question_answer 7) Select the true statement from the following. Nuclear force is
A)
strong, I short range and charge independent force
done
clear
B)
charge independent, attractive and long range force
done
clear
C)
strong, charge dependent and short range attractive force
done
clear
D)
long range, charge dependent and attractive force
done
clear
E)
charge independent, short range and strong repulsive force
done
clear
View Answer play_arrow
question_answer 8) In CE mode, the input characteristics of a transistor is the variation of
A)
\[{{I}_{B}}\] against \[{{V}_{BE}}\] at constant \[{{V}_{CE}}\]
done
clear
B)
\[{{I}_{C}}\] against \[{{V}_{CE}}\] at constant \[{{V}_{BE}}\]
done
clear
C)
\[{{I}_{B}}\] against \[{{I}_{C}}\]
done
clear
D)
\[{{I}_{E}}\]against \[{{I}_{C}}\]
done
clear
E)
\[{{I}_{C}}\] against \[{{V}_{CE}}\] at constant\[{{I}_{B}}\]
done
clear
View Answer play_arrow
question_answer 9) The electrical conductivity of an intrinsic semiconductor at OK is
A)
less than that of an insulator
done
clear
B)
is equal to zero
done
clear
C)
is equal to infinity
done
clear
D)
more than that of an insulator
done
clear
E)
is equal to that of a metal
done
clear
View Answer play_arrow
question_answer 10) The principle used for the transmission of light signals through the optical fibre is
A)
reflection
done
clear
B)
refraction
done
clear
C)
interference
done
clear
D)
diffraction
done
clear
E)
total internal reflection
done
clear
View Answer play_arrow
question_answer 11) The sky wave propagation is suitable for radio-waves of frequency
A)
upto 2 MHz
done
clear
B)
from 2 MHz to 20 MHz
done
clear
C)
from 2 MHz to 30 MHz
done
clear
D)
from 2 MHz to 50 MHz
done
clear
E)
from 2 MHz to 80 MHz
done
clear
View Answer play_arrow
question_answer 12) If voltage \[V=\left( 100\pm 5 \right)\] volt and current \[1=\left( 10\pm 0.2 \right)A,\] the percentage error in resistance R is
A)
5.2%
done
clear
B)
25%
done
clear
C)
7%
done
clear
D)
10%
done
clear
E)
2.5%
done
clear
View Answer play_arrow
question_answer 13) A body starting from rest moves with uniform acceleration. The distance covered by the body in time t is proportional to
A)
\[\sqrt{t}\]
done
clear
B)
\[{{t}^{3/2}}\]
done
clear
C)
\[{{t}^{2/3}}\]
done
clear
D)
\[{{t}^{3}}\]
done
clear
E)
\[{{t}^{2}}\]
done
clear
View Answer play_arrow
question_answer 14) A pendulum of length 1 m is released from\[\theta =60{}^\circ \]. The rate of change of speed of the bob at \[\theta =30{}^\circ \] is \[(g=10\,m{{s}^{-2}})\]
A)
\[10\,m{{s}^{-2}}\]
done
clear
B)
\[7.5\,m{{s}^{-2}}\]
done
clear
C)
\[5\,m{{s}^{-2}}\]
done
clear
D)
\[5\sqrt{3}\,m{{s}^{-2}}\]
done
clear
E)
\[2.5\,m{{s}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 15) A body constrained to move in the y-direction is subjected to a force\[\overrightarrow{F}=2\hat{i}+15\hat{j}+6\hat{k}\,N.\] The work done by this force in moving the body through a distance of 10 m along y-axis is
A)
100 J
done
clear
B)
150 J
done
clear
C)
120 J
done
clear
D)
200 J
done
clear
E)
50 J
done
clear
View Answer play_arrow
question_answer 16) A particle is projected with a speed v at \[45{}^\circ \] with the horizontal. The magnitude of angular momentum of the projectile about the point of projection when the particle is at its maximum height h is
A)
zero
done
clear
B)
\[\frac{mv{{h}^{2}}}{\sqrt{2}}\]
done
clear
C)
\[\frac{m{{v}^{2}}h}{2}\]
done
clear
D)
\[\frac{mv{{h}^{3}}}{\sqrt{2}}\]
done
clear
E)
\[\frac{mvh}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 17) A gun fires bullets each of mass 1 g with velocity of 10\[m{{s}^{-1}}\]by exerting a constant force of 5 g weight. Then the number of bullets fired per second is (Take g = 10\[m{{s}^{-1}}\])
A)
50
done
clear
B)
5
done
clear
C)
10
done
clear
D)
25
done
clear
E)
15
done
clear
View Answer play_arrow
question_answer 18) Two equal forces are acting at a point with an angle of \[60{}^\circ \] between them. If the resultant force is equal to \[40\sqrt{3}N,\] the magnitude of each force is
A)
40 N
done
clear
B)
20 N
done
clear
C)
80 N
done
clear
D)
30 N
done
clear
E)
10 N
done
clear
View Answer play_arrow
question_answer 19) A man pushes against a wall but fails to move it. He does
A)
negative work
done
clear
B)
positive but not maximum work
done
clear
C)
maximum positive work
done
clear
D)
no work at all
done
clear
E)
maximum negative work
done
clear
View Answer play_arrow
question_answer 20) When a bullet is fired at a target, its velocity decreases by half after penetrating 30 cm into it. The additional thickness it will penetrate before coming to rest is
A)
30 cm
done
clear
B)
40 cm
done
clear
C)
10 cm
done
clear
D)
50 cm
done
clear
E)
20 cm
done
clear
View Answer play_arrow
question_answer 21) The moment of inertia of a flywheel having kinetic energy 360 J and angular speed of 20 rad/s is
A)
\[18\,kg\text{-}{{m}^{2}}\]
done
clear
B)
\[1.8\,kg\text{-}{{m}^{2}}\]
done
clear
C)
\[2.5\,kg\text{-}{{m}^{2}}\]
done
clear
D)
\[9\,kg\text{-}{{m}^{2}}\]
done
clear
E)
\[0.9\,kg\text{-}{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 22) Pour point masses P, Q, R and S with respective masses 1 kg, 1 kg, 2 kg and 2 kg form the comers of a square of side a. The centre of mass of the system will be farthest from
A)
P only
done
clear
B)
R and S
done
clear
C)
R only
done
clear
D)
P and Q
done
clear
E)
P and R
done
clear
View Answer play_arrow
question_answer 23) The escape velocity from the earth is 11.2 km/s. The escape velocity from a planet having twice the radius and the same mean density is (in km/s)
A)
11.2
done
clear
B)
5.6
done
clear
C)
1.5
done
clear
D)
22.4
done
clear
E)
33.6
done
clear
View Answer play_arrow
question_answer 24) The excess of pressure inside the first soap bubble is three times that inside the second bubble. The ratio of volume of the first to that of the second bubble is
A)
1 : 3
done
clear
B)
1 : 9
done
clear
C)
1 : 27
done
clear
D)
9 : 1
done
clear
E)
27 : 1
done
clear
View Answer play_arrow
question_answer 25) Bernoullis principle is based on the law of conservation of
A)
mass
done
clear
B)
momentum
done
clear
C)
pressure
done
clear
D)
energy
done
clear
E)
volume
done
clear
View Answer play_arrow
question_answer 26)
A body of mass 8 kg is suspended through two light springs X and Y connected in series as shown in figure. The readings in X and Y respectively are
A)
8 kg, zero
done
clear
B)
zero, 8 kg
done
clear
C)
6 kg, 2 kg
done
clear
D)
2 kg, 6 kg
done
clear
E)
8 kg, 8 kg
done
clear
View Answer play_arrow
question_answer 27) A body cools from \[62{}^\circ \,C\] to \[50{}^\circ C\] in 10 min and to \[42{}^\circ C\] in the next 10 min. The temperature of the surrounding is
A)
\[16{}^\circ C\]
done
clear
B)
\[26{}^\circ C\]
done
clear
C)
\[36{}^\circ C\]
done
clear
D)
\[21{}^\circ C\]
done
clear
E)
\[31{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 28) At what temperature the kinetic energy of a gas molecule is half of the value at\[27{}^\circ C\]?
A)
\[13.5\,{}^\circ C\]
done
clear
B)
\[150{}^\circ C\]
done
clear
C)
\[75\,K\]
done
clear
D)
\[13.5\,K\]
done
clear
E)
\[-123{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 29) A black body emits radiations of maximum intensity for the wavelength of 5000\[\overset{\text{o}}{\mathop{\text{A}}}\,\] when the temperature of the body is \[1227\,{}^\circ C\]. If the temperature of the body is increased by \[1000{}^\circ C,\] the maximum intensity would be observed at
A)
1000 \[\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
2000 \[\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
5000 \[\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
4000 \[\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
E)
3000 \[\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 30) A body of mass 20 g connected to spring of constant k executes simple harmonic motion with a frequency of\[\left( \frac{5}{\pi } \right)Hz.\]. The value of spring constant is
A)
\[4\,N\text{-}{{m}^{-1}}\]
done
clear
B)
\[3\,N\text{-}{{m}^{-1}}\]
done
clear
C)
\[2\,N\text{-}{{m}^{-1}}\]
done
clear
D)
\[5\,N\text{-}{{m}^{-1}}\]
done
clear
E)
\[2.5\,N\text{-}{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 31) Two waves are given by \[{{y}_{1}}=\cos \,(4t-2x)\] and\[{{y}_{2}}=\sin \left( 4t-2x+\frac{\pi }{4} \right)\]. The phase difference between the two waves is
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[-\frac{\pi }{4}\]
done
clear
C)
\[\frac{3\pi }{4}\]
done
clear
D)
\[\frac{\pi }{2}\]
done
clear
E)
\[\frac{3\pi }{2}\]
done
clear
View Answer play_arrow
question_answer 32)
In the adjoining figure the equivalent resistance between A and B is
A)
50
done
clear
B)
80
done
clear
C)
2.50
done
clear
D)
6.80
done
clear
E)
7.80
done
clear
View Answer play_arrow
question_answer 33) In a Wheatstones network P = 2\[\Omega \], Q = 2\[\Omega \], R = 2\[\Omega \] and S = 3\[\Omega \]. The resistance with which S is to be shunted in order that the bridge may be balanced is
A)
1\[\Omega \]
done
clear
B)
2\[\Omega \]
done
clear
C)
4\[\Omega \]
done
clear
D)
6\[\Omega \]
done
clear
E)
8\[\Omega \]
done
clear
View Answer play_arrow
question_answer 34) A proton with energy of 2 MeV enters a uniform magnetic field of 2.5 T normally. The magnetic force on the proton is (Take mass of proton to be \[1.6\times {{10}^{-27}}kg\])
A)
\[3\times {{10}^{-12}}N\]
done
clear
B)
\[8\times {{10}^{-10}}N\]
done
clear
C)
\[8\times {{10}^{-12}}N\]
done
clear
D)
\[2\times {{10}^{-10}}N\]
done
clear
E)
\[3\times {{10}^{-10}}N\]
done
clear
View Answer play_arrow
question_answer 35) Two forces of 5N and 12 N. simultaneously act on a particle. The net force on the particle is
A)
17 N only
done
clear
B)
12 N
done
clear
C)
13 N
done
clear
D)
between 7N and 17 N
done
clear
E)
7 N only
done
clear
View Answer play_arrow
question_answer 36) If the acceleration due to gravity on the surface of earth of radius R is g, the gain in potential energy of a body of mass m raised from the surface to a height R is
A)
\[4\,mgR\]
done
clear
B)
\[mgR\text{/}4\]
done
clear
C)
\[mg\,R\text{/2}\]
done
clear
D)
\[2mgR\]
done
clear
E)
\[mgR\]
done
clear
View Answer play_arrow
question_answer 37) The core of a transformer is laminated to
A)
increase the magnetic flux linked
done
clear
B)
reduce the power loss due to eddy current
done
clear
C)
reduce the flux leakage loss
done
clear
D)
reduce copper loss
done
clear
E)
reduce hysteresis loss
done
clear
View Answer play_arrow
question_answer 38) Two stretched strings of same material are vibrating under same tension in fundamental mode. The ratio of their frequencies is 1: 2 and ratio of the length of the vibrating segments is 1 : 4. Then the ratio of the radii of the strings is
A)
2 : 1
done
clear
B)
4 : 1
done
clear
C)
3 : 2
done
clear
D)
8 : 1
done
clear
E)
4 : 5
done
clear
View Answer play_arrow
question_answer 39) Blood is flowing at the rate of 200 \[c{{m}^{3}}{{s}^{-1}}\] in a capillary of cross-sectional area 0.5 m. The velocity of flow (in mm\[{{s}^{-1}}\]) is
A)
0.1
done
clear
B)
0.2
done
clear
C)
0.3
done
clear
D)
0.4
done
clear
E)
0.5
done
clear
View Answer play_arrow
question_answer 40) The electrical conductivity of a semiconductor increases when an electromagnetic radiation of wavelength shorter than 1125 nm is incident on it. The band gap of the semiconductor is
A)
0.9 eV
done
clear
B)
0.7 eV
done
clear
C)
0.5 eV
done
clear
D)
0.8 eV
done
clear
E)
1.1 eV
done
clear
View Answer play_arrow
question_answer 41) 4 cells each of emf 2V and internal resistance of 1\[\Omega \] are connected in parallel to a load resistor of 2\[\Omega \]. Then the current through the load resistor is
A)
2 A
done
clear
B)
1.5 A
done
clear
C)
1A
done
clear
D)
0.888 A
done
clear
E)
0.75 A
done
clear
View Answer play_arrow
question_answer 42) A thermos flask made of stainless steel contains several tiny lead shots. If the flask is quickly shaken up and down several times, the temperature of lead shots
A)
increases by adiabatic process
done
clear
B)
increases by isothermal process
done
clear
C)
decreases by adiabatic process
done
clear
D)
remains same
done
clear
E)
first decreases and then increases
done
clear
View Answer play_arrow
question_answer 43) The slope of the kinetic energy versus position vector gives the rate of change of
A)
momentum
done
clear
B)
velocity
done
clear
C)
force
done
clear
D)
power
done
clear
E)
work
done
clear
View Answer play_arrow
question_answer 44) The total electrical flux leaving a spherical surface of radius r, in enclosing an electric dipole of charge q is
A)
zero
done
clear
B)
\[\frac{q}{{{\varepsilon }_{0}}}\]
done
clear
C)
\[\frac{8\pi {{r}^{2}}q}{{{\varepsilon }_{0}}}\]
done
clear
D)
\[\frac{2q}{{{\varepsilon }_{0}}}\]
done
clear
E)
\[\frac{4\pi {{r}^{2}}q}{{{\varepsilon }_{0}}}\]
done
clear
View Answer play_arrow
question_answer 45) A current \[I=100\,\sin \,(100\pi t)A\] is passed in coil, which induces a maximum emf 5\[\pi \]volt in second coil. The mutual inductance between the coils is
A)
10 mH
done
clear
B)
15 mH
done
clear
C)
25 mH
done
clear
D)
20 mH
done
clear
E)
5 mH
done
clear
View Answer play_arrow
question_answer 46) What is the effect on the time period of a simple pendulum, if the mass of the bob is doubled?
A)
Halved
done
clear
B)
Doubled
done
clear
C)
Becomes eight times
done
clear
D)
Becomes zero
done
clear
E)
No effect
done
clear
View Answer play_arrow
question_answer 47) The half-life of thorium X is 3.64 days. After how many days will 0.1 of the mass of a sample of the substance remain undecayed?
A)
12.1 days
done
clear
B)
24 days
done
clear
C)
60 days
done
clear
D)
4 days
done
clear
E)
14 days
done
clear
View Answer play_arrow
question_answer 48) Moment of inertia of a body does not depend upon its
A)
mass
done
clear
B)
axis of rotation
done
clear
C)
shape
done
clear
D)
distribution of mass
done
clear
E)
angular velocity
done
clear
View Answer play_arrow
question_answer 49) In terms of Bohr radius \[{{a}_{0}}\] the radius of second Bohr orbit of hydrogen atom is given by
A)
\[4\,{{a}_{0}}\]
done
clear
B)
\[8\,{{a}_{0}}\]
done
clear
C)
\[\sqrt{2}\,{{a}_{0}}\]
done
clear
D)
\[2\,{{a}_{0}}\]
done
clear
E)
\[\sqrt{4}\,{{a}_{0}}\]
done
clear
View Answer play_arrow
question_answer 50) What is the escape velocity for a body on it surface of a planet on which the accelerant due to gravity is \[{{(3.1)}^{2}}m{{s}^{-2}}\]and whose radius is 8100 km?
A)
\[2790\,km\text{-}{{s}^{-1}}\]
done
clear
B)
\[27.9\,km\text{-}{{s}^{-1}}\]
done
clear
C)
\[\frac{27.9}{\sqrt{5}}\,km\text{-}{{s}^{-1}}\]
done
clear
D)
\[27.9\sqrt{5}\,km\text{-}{{s}^{-1}}\]
done
clear
E)
\[\frac{2.79}{\sqrt{5}}km\text{-}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 51) Which one of the following is not a derived unit?
A)
Frequency
done
clear
B)
Plancks constant
done
clear
C)
Gravitational constant
done
clear
D)
Charge
done
clear
E)
Electric current
done
clear
View Answer play_arrow
question_answer 52) The diagonals of a parallelogram represented by vectors\[\overrightarrow{P}=5\hat{i}-4\hat{j}+3\hat{k}\]and \[\overrightarrow{q}=3\hat{i}+2\hat{j}-\hat{k}.\] Then the area of the parallelogram is
A)
\[\sqrt{171}\,\text{unit}\]
done
clear
B)
\[\sqrt{72}\,\text{unit}\]
done
clear
C)
\[171\,\,\text{unit}\]
done
clear
D)
\[\sqrt{191}\,\,\text{unit}\]
done
clear
E)
None of the above
done
clear
View Answer play_arrow
question_answer 53) The correct arrangement of colours in the descending order of their wavelength is
A)
yellow, violet, green, orange
done
clear
B)
orange, yellow, green, violet
done
clear
C)
violet, green, yellow, orange
done
clear
D)
yellow, green, orange, violet
done
clear
E)
orange, green, violet, yellow
done
clear
View Answer play_arrow
question_answer 54) The velocity of sound is v at 273K. The temperature at which it is 2v is
A)
\[2\times 273\,K\]
done
clear
B)
\[4\times 273\,K\]
done
clear
C)
\[8\times 273\,K\]
done
clear
D)
\[16\times 273\,K\]
done
clear
E)
\[\sqrt{2}\times 273\,K\]
done
clear
View Answer play_arrow
question_answer 55) Same force acts on two bodies of different masses 3 kg and 5 kg initially at rest. The ratio of times required to acquire same final velocity is
A)
\[5:3\]
done
clear
B)
\[25:9\]
done
clear
C)
\[9:25\]
done
clear
D)
\[\sqrt{3}:\sqrt{5}\]
done
clear
E)
\[3:5\]
done
clear
View Answer play_arrow
question_answer 56) To decrease the volume of a gas by 5% at constant temperature the pressure should be
A)
decreased by 5.26%
done
clear
B)
increased by 5.2%
done
clear
C)
decreased by 11%
done
clear
D)
increased by 11%
done
clear
E)
increased by 15%
done
clear
View Answer play_arrow
question_answer 57) A block of wood weight 4 N in air and 3N, when immersed in a liquid. The buoyant force in newton is
A)
zero
done
clear
B)
1
done
clear
C)
3/4
done
clear
D)
4/3
done
clear
E)
7
done
clear
View Answer play_arrow
question_answer 58) A 16\[\mu F\] capacitor is charged to a 20V potential. The battery is then disconnected and pure 40 mH coil is connected across the capacitor, so that LC oscillations are setup. The maximum current in the coil is
A)
0.2 A
done
clear
B)
40 mA
done
clear
C)
2 A
done
clear
D)
0.4 A
done
clear
E)
0.8 A
done
clear
View Answer play_arrow
question_answer 59) A concave lens of focal length 20 cm produces an image half the size of the real object. The distance of the real object is
A)
20 cm
done
clear
B)
30 cm
done
clear
C)
10 cm
done
clear
D)
60 cm
done
clear
E)
40 cm
done
clear
View Answer play_arrow
question_answer 60) If the kinetic energy of the particle is increased by 16 times, the percentage change in the de-Broglie wavelength of the particle is
A)
25%
done
clear
B)
75%
done
clear
C)
60%
done
clear
D)
50%
done
clear
E)
30%
done
clear
View Answer play_arrow
question_answer 61) Which one of the following is a tridentate ligand?
A)
\[NO_{2}^{-}\]
done
clear
B)
Oxalate ion
done
clear
C)
Glycinate ion
done
clear
D)
Dien
done
clear
E)
EDTA
done
clear
View Answer play_arrow
question_answer 62) For the purification, isolation and separation of organic compounds, the latest technique followed is
A)
chromatography
done
clear
B)
steam distillation
done
clear
C)
fractional crystallization
done
clear
D)
sublimation
done
clear
E)
vacuum distillation
done
clear
View Answer play_arrow
question_answer 63) Which one of the following compounds cannot show tautomerism?
A)
\[C{{H}_{3}}-\underset{O}{\mathop{\underset{\mathbf{||}}{\mathop{C}}\,}}\,-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{2}}=CH-OH\]
done
clear
C)
done
clear
D)
done
clear
E)
done
clear
View Answer play_arrow
question_answer 64)
The reaction,
A)
Wurtz reaction
done
clear
B)
Wittig reaction
done
clear
C)
Ullmann reaction
done
clear
D)
Williamson reaction
done
clear
E)
Wurtz-Fittig reaction
done
clear
View Answer play_arrow
question_answer 65) Chloroform gives a trichloro derivative of an alcohol on reaction with
A)
cone. nitric acid
done
clear
B)
aq. Alkali
done
clear
C)
acetone and alkali
done
clear
D)
sodium ethoxide
done
clear
E)
a primary amine and an alkali
done
clear
View Answer play_arrow
question_answer 66) \[10\,L\] of\[{{O}_{2}}\]gas is reacted with 30 L of CO gas at STP. The volumes of each gas present at the end of the reaction are
A)
\[CO=10\,L,\,C{{O}_{2}}=20L\]
done
clear
B)
\[{{O}_{2}}=10\,L,\,CO=20L\]
done
clear
C)
\[CO=20\,L,\,C{{O}_{2}}=10L\]
done
clear
D)
\[{{O}_{2}}=10\,L,\,C{{O}_{2}}=20L\]
done
clear
E)
\[{{O}_{2}}=10\,L,\,CO=20L\]
done
clear
View Answer play_arrow
question_answer 67) For an atom, having \[n=4,\]\[{{m}_{l}}=+1,\]the maximum number of electrons is
A)
4
done
clear
B)
15
done
clear
C)
3
done
clear
D)
1
done
clear
E)
6
done
clear
View Answer play_arrow
question_answer 68) Which of the following diagrams correctly describes the behaviour of a fixed mass of an ideal gas? (T is measured in K).
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
E)
done
clear
View Answer play_arrow
question_answer 69) The hybridisation of oxygen atom in \[{{H}_{2}}{{O}_{2}}\] is
A)
\[s{{p}^{3}}d\]
done
clear
B)
\[sp\]
done
clear
C)
\[s{{p}^{2}}\]
done
clear
D)
\[s{{p}^{3}}\]
done
clear
E)
\[s{{p}^{3}}{{d}^{2}}\]
done
clear
View Answer play_arrow
question_answer 70) Among the metals Cr, Fe, Mn, Ti, Zn and Mg, the one that cannot be obtained by reduction of its metal oxide by aluminium is
A)
Cr
done
clear
B)
Fe
done
clear
C)
Mn
done
clear
D)
Zn
done
clear
E)
Mg
done
clear
View Answer play_arrow
question_answer 71) For which one of the following minerals, the composition given is incorrect?
A)
Glaubers salt - \[N{{a}_{2}}S{{O}_{4}}\cdot 10\,{{H}_{2}}O\]
done
clear
B)
Borax - \[N{{a}_{2}}{{B}_{4}}{{O}_{7}}\cdot 7{{H}_{2}}O\]
done
clear
C)
Camallite - \[KCl\cdot MgC{{l}_{2}}\cdot 6{{H}_{2}}O\]
done
clear
D)
Soda ash - \[N{{a}_{2}}C{{O}_{3}}\]
done
clear
E)
Epsom salt - \[MgS{{O}_{4}}\cdot 7{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 72) Which of the following is the correct order of increasing enthalpy of vaporisation?
A)
\[N{{H}_{3}}<P{{H}_{3}}<As{{H}_{3}}\]
done
clear
B)
\[As{{H}_{3}}<P{{H}_{3}}<N{{H}_{3}}\]
done
clear
C)
\[P{{H}_{3}}<As{{H}_{3}}<N{{H}_{3}}\]
done
clear
D)
\[N{{H}_{3}}<As{{H}_{3}}<P{{H}_{3}}\]
done
clear
E)
\[As{{H}_{3}}<N{{H}_{3}}<P{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 73) Chlorine reacts with excess of ammonia to form
A)
\[N{{H}_{4}}Cl\]
done
clear
B)
\[{{N}_{2}}+HCl\]
done
clear
C)
\[{{N}_{2}}+N{{H}_{4}}Cl\]
done
clear
D)
\[{{N}_{2}}+NC{{l}_{3}}\]
done
clear
E)
\[NC{{l}_{3}}+HCl\]
done
clear
View Answer play_arrow
question_answer 74) The correct order of ionic radii of \[{{Y}^{3+}},\]\[L{{a}^{3+}},\]\[E{{u}^{3+}}\]and \[L{{u}^{3+}}\] is
A)
\[{{Y}^{3+}}<L{{a}^{3+}}<E{{u}^{3+}}<L{{u}^{3+}}\]
done
clear
B)
\[L{{u}^{3+}}<E{{u}^{3+}}<L{{a}^{3+}}<{{Y}^{3+}}\]
done
clear
C)
\[L{{a}^{3+}}<E{{u}^{3+}}<L{{u}^{3+}}<{{Y}^{3+}}\]
done
clear
D)
\[{{Y}^{3+}}<L{{u}^{3+}}<E{{u}^{3+}}<L{{a}^{3+}}\]
done
clear
E)
\[E{{u}^{3+}}<L{{a}^{3+}}<L{{u}^{3+}}<{{Y}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 75) The bond dissociation energies of \[{{H}_{2}},\,C{{l}_{2}}\] and \[HCl\] are 104, 58 and 103 kcal\[\text{mo}{{\text{l}}^{-1}}\] respectively. The enthalpy of formation of \[HCl\]would be
A)
\[-\,22\text{ kcal mo}{{\text{l}}^{-1}}\]
done
clear
B)
\[-\,44\text{ kcal mo}{{\text{l}}^{-1}}\]
done
clear
C)
\[+\,44\text{ kcal mo}{{\text{l}}^{-1}}\]
done
clear
D)
\[+\,22\text{ kcal mo}{{\text{l}}^{-1}}\]
done
clear
E)
\[-\,11\text{ kcal mo}{{\text{l}}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 76) The vant Hoff factor for a solute that associates in solution is
A)
zero
done
clear
B)
1.0
done
clear
C)
less than 1
done
clear
D)
more than 1
done
clear
E)
between 1 and 2
done
clear
View Answer play_arrow
question_answer 77) Which of the following is redox reaction?
A)
\[2CuS{{O}_{4}}+4KI\to C{{u}_{2}}{{I}_{2}}+2{{K}_{2}}S{{O}_{4}}+{{I}_{2}}\]
done
clear
B)
\[S{{O}_{2}}+{{H}_{2}}O\to {{H}_{2}}S{{O}_{3}}\]
done
clear
C)
\[N{{a}_{2}}S{{O}_{4}}+BaC{{l}_{2}}\to BaS{{O}_{4}}+2NaCl\]
done
clear
D)
\[CuS{{O}_{4}}+4N{{H}_{3}}\to [Cu{{(N{{H}_{3}})}_{4}}]S{{O}_{4}}\]
done
clear
E)
\[{{C}_{12}}{{H}_{22}}{{O}_{11}}+{{H}_{2}}O\to {{C}_{6}}{{H}_{12}}{{O}_{6}}+{{C}_{6}}{{H}_{12}}{{O}_{6}}\]
done
clear
View Answer play_arrow
question_answer 78) The resistance of N/10 solution is found to be \[2.5\times {{10}^{3}}\]ohm. The equivalent conductance of the solution is (cell constant = 1.25\[c{{m}^{-1}}\])
A)
\[2.5\,\,oh{{m}^{-1}}c{{m}^{2}}equi{{v}^{-1}}\]
done
clear
B)
\[5.0\,\,oh{{m}^{-1}}c{{m}^{2}}equi{{v}^{-1}}\]
done
clear
C)
\[2.5\,\,oh{{m}^{-1}}c{{m}^{2}}equi{{v}^{-1}}\]
done
clear
D)
\[5.0\,\,oh{{m}^{-1}}c{{m}^{2}}equi{{v}^{-1}}\]
done
clear
E)
\[1.25\,\,oh{{m}^{-1}}c{{m}^{2}}equi{{v}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 79) Which one of the following acts as the best coagulating agents for ferric hydroxide sol?
A)
Magnesium chloride
done
clear
B)
Hydrochloric acid
done
clear
C)
Aluminium chloride
done
clear
D)
Potassium oxalate
done
clear
E)
Potassium ferricyanide
done
clear
View Answer play_arrow
question_answer 80) If the equilibrium constant for the reaction \[{{N}_{2}}(g)+3{{H}_{2}}(g)\rightleftharpoons 2N{{H}_{3}}(g)\] at 750 K is 49, then the equilibrium constant for the reaction \[N{{H}_{3}}(g)\rightleftharpoons \frac{1}{2}{{N}_{2}}(g)+\frac{3}{2}{{H}_{2}}(g)\]at the same temperature is
A)
1/49
done
clear
B)
49
done
clear
C)
7
done
clear
D)
\[{{49}^{2}}\]
done
clear
E)
1/7
done
clear
View Answer play_arrow
question_answer 81) The correct relation between equilibrium constant (K), standard free energy\[(\Delta G{}^\circ )\]and temperature (T) is
A)
\[\Delta G{}^\circ =RT\,\ln K\]
done
clear
B)
\[K={{e}^{-\Delta G{}^\circ /2303RT}}\]
done
clear
C)
\[\Delta G{}^\circ =-RT{{\log }_{10}}K\]
done
clear
D)
\[K={{10}^{-\Delta G{}^\circ /2.303\,RT}}\]
done
clear
E)
\[\Delta G{}^\circ =R\,\ln \,K\]
done
clear
View Answer play_arrow
question_answer 82) Which among the following is the heaviest?
A)
1 mole of oxygen
done
clear
B)
1 molecule of sulphur trioxide
done
clear
C)
100 u of uranium
done
clear
D)
10 moles of hydrogen
done
clear
E)
44 g of carbon dioxide
done
clear
View Answer play_arrow
question_answer 83) Which one of the following electrolytes would dissolve in water to give an alkaline solution?
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}COONa\]
done
clear
C)
\[N{{H}_{4}}Cl\]
done
clear
D)
\[HCN\]
done
clear
E)
\[NaCl\]
done
clear
View Answer play_arrow
question_answer 84) The efficiency of an enzyme to catalyse a reaction is due to its capacity to
A)
reduce the activation energy of the reaction
done
clear
B)
form strong enzyme-substrate complex
done
clear
C)
decrease the bond energy of all substrate molecules
done
clear
D)
increase the free energy of the catalyst-substrate reaction
done
clear
E)
alter the substrate geometry to fit into the shape of the enzyme molecule
done
clear
View Answer play_arrow
question_answer 85) If 0.5 g of a solute (molar mass 100 g\[mo{{l}^{-1}}\]) in of solvent elevates the boiling point by 1K, the molar boiling point constant of the solvent is
A)
2
done
clear
B)
8
done
clear
C)
5
done
clear
D)
0.5
done
clear
E)
10
done
clear
View Answer play_arrow
question_answer 86) \[A\xrightarrow{Dil\,NaOH}{{(C{{H}_{3}})}_{2}}C=CHOCH=C{{(C{{H}_{3}})}_{2}}\]What is A?
A)
Acetone
done
clear
B)
Acetaldehyde
done
clear
C)
Propionaldehyde
done
clear
D)
Formaldehyde
done
clear
E)
Ethyl alcohol
done
clear
View Answer play_arrow
question_answer 87) Which of the following metals, Fe, Zn, Pb, Ag and Pt, do not give a metal nitrate on treatment with concentrated\[HN{{O}_{3}}\]?
A)
Fe and Zn
done
clear
B)
Fe and Pt
done
clear
C)
Pb, Ag and Pt
done
clear
D)
Fe, Ag and Pt
done
clear
E)
Fe, Zn and Pt
done
clear
View Answer play_arrow
question_answer 88) One of the different amino acids which can be synthesized in the body is
A)
lysine
done
clear
B)
leucine
done
clear
C)
valine
done
clear
D)
phenyl alanine
done
clear
E)
alanine
done
clear
View Answer play_arrow
question_answer 89) Neopentyl bromide undergoes dehydro halogenation to give alkene even though it has no \[\beta \text{-}\]hydrogen. This is due to
A)
E2 mechanism
done
clear
B)
E1 mechanism
done
clear
C)
due to rearrangement of carbocation by E1 mechanism
done
clear
D)
E1 CB mechanism
done
clear
E)
Hermann elimination
done
clear
View Answer play_arrow
question_answer 90) Which of the following statements is true?
A)
The kinetic energy of an electron is inversely proportional to square of its momentum
done
clear
B)
de-Broglie wavelength associated with a particle is directly proportional to its mass
done
clear
C)
de-Broglie wavelength associated with a particle is directly proportional to square of its velocity
done
clear
D)
The wavelength associated with an electron is directly proportional to square root of accelerating potential
done
clear
E)
The kinetic energy of an electron is directly proportional to accelerating potential
done
clear
View Answer play_arrow
question_answer 91) Dead burnt plaster is
A)
\[CaS{{O}_{4}}\cdot 2{{H}_{2}}O\]
done
clear
B)
\[MgS{{O}_{4}}\cdot 7{{H}_{2}}O\]
done
clear
C)
\[CaS{{O}_{4}}\cdot 1/2\,{{H}_{2}}O\]
done
clear
D)
\[CaS{{O}_{4}}\]
done
clear
E)
\[MgS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 92) The diketone\[C{{H}_{3}}-\overset{O}{\mathop{\overset{\mathbf{||}}{\mathop{C}}\,}}\,-{{(C{{H}_{2}})}_{2}}-\overset{O}{\mathop{\overset{\mathbf{||}}{\mathop{C}}\,}}\,-C{{H}_{3}}\]on intramolecular aldol condensation gives the final product
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
E)
done
clear
View Answer play_arrow
question_answer 93) The electronic configuration of four elements are 1. \[[Xe]\,6{{s}^{1}}\] 2. \[[Xe]\,4{{f}^{14}},5{{d}^{1}},6{{s}^{2}}\] 3. \[[\text{Ar}]\,4{{s}^{2}}4{{p}^{5}}\] 4. \[[\text{Ar}]\,3{{d}^{7}},4{{s}^{2}}\] Which one of the following statements about these elements is not correct?
A)
1 is a strong reducing agent
done
clear
B)
2 is a d-block element
done
clear
C)
3 has high electron affinity
done
clear
D)
4 shows variable oxidation state
done
clear
E)
The compound formed between 1 and 3 is ionic
done
clear
View Answer play_arrow
question_answer 94) Which one of the following statements about \[C{{H}_{3}}CN\] is not true?
A)
Its IUPAC name is ethane nitrile
done
clear
B)
The bond between C and N is a triple bond
done
clear
C)
The C -C - N bond angle is \[180{}^\circ \]
done
clear
D)
The carbon-carbon bond is longer than the carbon-nitrogen bond
done
clear
E)
It has a relatively high boiling point due to hydrogen bonding
done
clear
View Answer play_arrow
question_answer 95) For the homogeneous reaction\[xA+yB\rightleftharpoons lY+mZ\]\[\Delta H{}^\circ =-30\,kJ\,mo{{l}^{-1}}\]and \[\Delta S=-100\,JK\,mo{{l}^{-1}}\] At what temperature the reaction is at equilibrium?
A)
\[50{}^\circ C\]
done
clear
B)
\[250{}^\circ C\]
done
clear
C)
\[100K\]
done
clear
D)
\[27{}^\circ C\]
done
clear
E)
\[500K\]
done
clear
View Answer play_arrow
question_answer 96) The half cell reaction with their standard electrode potentials are \[P{{b}^{2+}}(aq)+2{{e}^{-}}t\xrightarrow{{}}Pb(s);\]\[E{}^\circ =-\,0.13\,V\] \[A{{g}^{+}}(aq)+{{e}^{-}}\xrightarrow{{}}Ag(s);\] \[E{}^\circ =+\,0.80\,V\] What is the emf of the cell?
A)
\[-\,0.93\,\,V\]
done
clear
B)
\[+\,0.93\,V\]
done
clear
C)
\[+\,0.67\,V\]
done
clear
D)
\[-\,0.67\,\,V\]
done
clear
E)
None of these
done
clear
View Answer play_arrow
question_answer 97) Which of the following molecule is planar?
A)
\[C{{H}_{4}}\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
D)
\[SiC{{l}_{4}}\]
done
clear
E)
\[PC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 98) The formula of compound which gives violet colour in Lassaignes test for sulphur with sodium nitroprusside is
A)
\[N{{a}_{4}}[Fe{{(CN)}_{6}}S]\]
done
clear
B)
\[N{{a}_{4}}[Fe{{(CN)}_{5}}NCS]\]
done
clear
C)
\[N{{a}_{4}}[Fe{{(CN)}_{5}}NOS]\]
done
clear
D)
\[N{{a}_{2}}[Fe{{(CN)}_{5}}NOS]\]
done
clear
E)
\[N{{a}_{4}}[Fe{{(CN)}_{5}}S]\]
done
clear
View Answer play_arrow
question_answer 99) The strongest ortho-para and strongest mete-directing groups respectively, are
A)
\[-N{{O}_{2}}\] and\[-N{{H}_{2}}\]
done
clear
B)
\[-CON{{H}_{2}}\] and\[-N{{H}_{2}}\]
done
clear
C)
\[-N{{H}_{2}}\] and\[-CON{{H}_{2}}\]
done
clear
D)
\[-X\] and\[-CON{{H}_{2}}\]
done
clear
E)
\[-N{{H}_{2}}\] and\[-N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 100) When excess ammonia is added to \[CuS{{O}_{4}}\] solution the deep blue complex obtained is
A)
tetrahedral and paramagnetic
done
clear
B)
tetrahedral and diamagnetic
done
clear
C)
square planar and diamagnetic
done
clear
D)
square planar and paramagnetic
done
clear
E)
tetrahedral and ferromagnetic
done
clear
View Answer play_arrow
question_answer 101) The angular momentum of an electron is zero. In which orbital may it be present?
A)
2s
done
clear
B)
2p
done
clear
C)
3d
done
clear
D)
4f
done
clear
E)
5f
done
clear
View Answer play_arrow
question_answer 102) Which of the following will show geometrical isomerism?
A)
2-methyl butene
done
clear
B)
Propene
done
clear
C)
Vinyl chloride
done
clear
D)
But-2-ene
done
clear
E)
2-methyl propene
done
clear
View Answer play_arrow
question_answer 103) A substance with initial concentration of a mol \[d{{m}^{-3}}\]reacts according to zero order kinetics. The time it takes for the completion of the reaction is: (k = rate constant)
A)
\[k\text{/}a\]
done
clear
B)
\[a\text{/}2k\]
done
clear
C)
\[a\text{/}k\]
done
clear
D)
\[2k\text{/}a\]
done
clear
E)
\[ka\]
done
clear
View Answer play_arrow
question_answer 104) Biotin is an organic compound present in yeast. Its deficiency in diet causes dermatitis and paralysis. It is also known as
A)
Vitamin \[H\]
done
clear
B)
Vitamin\[{{B}_{3}}\]
done
clear
C)
Vitamin \[{{B}_{12}}\]
done
clear
D)
Vitamin\[D\]
done
clear
E)
Vitamin \[E\]
done
clear
View Answer play_arrow
question_answer 105) Consider the following reaction at\[1000{}^\circ C\]. (I) \[Zn(s)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}ZnO(s);\]\[\Delta G{}^\circ =-360\,kJ\,mo{{l}^{-1}}\] (II) \[C\text{(graphite)}+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}CO(g);\]\[\Delta G{}^\circ =-\,460\,kJ\,mo{{l}^{-1}}\] Choose the correct statement at\[1000{}^\circ C\].
A)
Zinc can be oxidised by carbon monoxide
done
clear
B)
Zinc oxide can be reduced by graphite
done
clear
C)
Both statements a and b are true
done
clear
D)
Both statements a and b are false
done
clear
E)
Carbon monoxide can be reduced by zinc
done
clear
View Answer play_arrow
question_answer 106) Which of the following metals in solution forms a precipitate with\[NaOH,\]which is not soluble in an excess of the base?
A)
Fe
done
clear
B)
Sn
done
clear
C)
Pb
done
clear
D)
Zn
done
clear
E)
Al
done
clear
View Answer play_arrow
question_answer 107) The following is known as Bordeaux mixture.
A)
borax and copper sulphate
done
clear
B)
orthoboric acid and ferrous sulphate
done
clear
C)
sodium borate and zinc sulphate
done
clear
D)
copper sulphate and lime
done
clear
E)
borax and manganous sulphate
done
clear
View Answer play_arrow
question_answer 108) Which among the following will give a precipitate with ammoniacal silver nitrate?
A)
2-butene
done
clear
B)
2-butyne
done
clear
C)
Chlorobenzene
done
clear
D)
3-methyl-1-butyne
done
clear
E)
1, 3-butadiene
done
clear
View Answer play_arrow
question_answer 109)
Consider the following representations
A)
enantiomers
done
clear
B)
diastereomers
done
clear
C)
conformational isomers
done
clear
D)
identical
done
clear
E)
cis-trans isomers
done
clear
View Answer play_arrow
question_answer 110) Which one of the following compounds is used as a body deodorant?
A)
Aspirin
done
clear
B)
Omeprazole
done
clear
C)
Indigosol-O
done
clear
D)
p-chlorometaxylenol
done
clear
E)
Bithional
done
clear
View Answer play_arrow
question_answer 111) The rate constant for a first order reaction becomes six times when the temperature is raised from 350 K to 400 K. Calculate the activation energy for the reaction. \[(R=8.314\,\,J{{K}^{-1}}mo{{l}^{-1}})\]
A)
\[41.7\,kJ\,mo{{l}^{-1}}\]
done
clear
B)
\[4.17\,kJ\,mo{{l}^{-1}}\]
done
clear
C)
\[417\,kJ\,mo{{l}^{-1}}\]
done
clear
D)
\[0.417\,kJ\,mo{{l}^{-1}}\]
done
clear
E)
\[4170\,kJ\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 112) Which of the following is not a green house gas?
A)
\[C{{O}_{2}}\]
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[{{O}_{3}}\]
done
clear
D)
\[{{N}_{2}}\]
done
clear
E)
\[CC{{l}_{2}}{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 113) KBr is 80% dissociated in aqueous solution of 0.5m concentration. (Given,\[{{k}_{f}}\]for water = 1.86 K kg \[\text{mo}{{\text{l}}^{-1}}\]). The solution freezes at
A)
271.326 K
done
clear
B)
272 K
done
clear
C)
270.5 K
done
clear
D)
268.5 K
done
clear
E)
269 K
done
clear
View Answer play_arrow
question_answer 114) \[{}^{39}A{{r}_{18}}\] and \[{}^{40}{{K}_{19}}\] are
A)
isotopes
done
clear
B)
isobars
done
clear
C)
isotones
done
clear
D)
isosters
done
clear
E)
isodiaphers
done
clear
View Answer play_arrow
question_answer 115) Which pair of the gases diffuses with the same rate at same temperature and pressure?
A)
\[CO\] and \[NO\]
done
clear
B)
\[N{{O}_{2}}\] and \[C{{O}_{2}}\]
done
clear
C)
\[N{{H}_{3}}\] and \[P{{H}_{3}}\]
done
clear
D)
\[NO\] and \[{{C}_{2}}{{H}_{6}}\]
done
clear
E)
\[C{{l}_{2}}\] and \[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 116) In the Victor-Meyers test, the colours given by \[1{}^\circ ,\,\,2{}^\circ \] and \[3{}^\circ \]alcohols are respectively
A)
red, colourless, blue
done
clear
B)
red, blue, colourless
done
clear
C)
colourless, red, blue
done
clear
D)
red, blue, violet
done
clear
E)
blue, red, violet
done
clear
View Answer play_arrow
question_answer 117) A particular solid is very hard and has a high melting point. In solid state it is a non -conductor and its melt is a conductor of electricity. Classify the solid
A)
metallic
done
clear
B)
molecular
done
clear
C)
network
done
clear
D)
ionic
done
clear
E)
amorphous.
done
clear
View Answer play_arrow
question_answer 118) Which one of the following statements about diborane is not true?
A)
The B atoms in it are \[s{{p}^{3}}\] hybridized
done
clear
B)
It contains two 3-centre-2-electron bonds
done
clear
C)
All B-H bond lengths in it are equal due to resonance
done
clear
D)
The molecule is non-planar
done
clear
E)
The molecule contains 12 valence electrons
done
clear
View Answer play_arrow
question_answer 119) Which of the following is not aromatic?
A)
Benzene
done
clear
B)
Cyclopropenyl cation
done
clear
C)
Tropylium cation
done
clear
D)
Cyclopentadienyl cation
done
clear
E)
Cyclopentadienyl anion
done
clear
View Answer play_arrow
question_answer 120) The element that does not exhibit positive oxidation state is
A)
Fe
done
clear
B)
Cl
done
clear
C)
O
done
clear
D)
N
done
clear
E)
F
done
clear
View Answer play_arrow
question_answer 121) Which one is related to urine concentration in mammals?
A)
Testosterone hormone
done
clear
B)
Antidiuretic hormone
done
clear
C)
Oxytocin hormone
done
clear
D)
Insulin
done
clear
E)
All of the above
done
clear
View Answer play_arrow
question_answer 122) Adjacent epithelial cells are held together by means of
A)
microsomes
done
clear
B)
liposomes
done
clear
C)
desmosomes
done
clear
D)
glyoxysomes
done
clear
E)
None of these
done
clear
View Answer play_arrow
question_answer 123) First \[C{{O}_{2}}\] acceptor in \[{{C}_{4}}\]-plants is
A)
PEP
done
clear
B)
PGA
done
clear
C)
RuBP
done
clear
D)
pyruvic acid
done
clear
E)
OAA
done
clear
View Answer play_arrow
question_answer 124) In the members of family-Malvaceae anthers are described as
A)
diadelphous and dithecous
done
clear
B)
diadelphous and monothecous
done
clear
C)
monoadelphous and monothecous
done
clear
D)
monoadelphous and dithecous
done
clear
E)
None of the above
done
clear
View Answer play_arrow
question_answer 125) In the angiosperm ovule, central cell of the embryo sac, prior to the entry of pollen tube, contains
A)
a single haploid nucleus
done
clear
B)
one diploid and one haploid nuclei
done
clear
C)
two haploid polar nuclei
done
clear
D)
one diploid secondary nucleus
done
clear
E)
two diploid polar nuclei
done
clear
View Answer play_arrow
question_answer 126) Which type of cells are absent in sponges?
A)
Trophocytes
done
clear
B)
Myocytes
done
clear
C)
Archeocytes
done
clear
D)
Cnidocytes
done
clear
E)
All of these
done
clear
View Answer play_arrow
question_answer 127) Which of the following is purely motor cranial nerve?
A)
Optic
done
clear
B)
Olfactory
done
clear
C)
Vagus
done
clear
D)
Abducens
done
clear
E)
All of these
done
clear
View Answer play_arrow
question_answer 128) Which one of the following is correctly matched?
A)
Body louse - Typhoid
done
clear
B)
House fly - Yellow fever
done
clear
C)
Anopheles - Malaria
done
clear
D)
Aedes - Plague
done
clear
E)
Leishmania - Sysphilis
done
clear
View Answer play_arrow
question_answer 129) In earthworm self-fertilization cannot occur due to
A)
protogyny
done
clear
B)
protandry
done
clear
C)
epigyny
done
clear
D)
hypogyny
done
clear
E)
None of these
done
clear
View Answer play_arrow
question_answer 130) Which of the following is an opiate narcotic?
A)
Morphine
done
clear
B)
LSD
done
clear
C)
Amphetamines
done
clear
D)
Barbiturates
done
clear
E)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 131) Pollination by snail and slug is known as
A)
entomophilous
done
clear
B)
ornithophilous
done
clear
C)
anemophilous
done
clear
D)
malacophilous
done
clear
E)
hydrophilous
done
clear
View Answer play_arrow
question_answer 132) Which is the example of conditioned reflex?
A)
Your kneeing took up a stone than dog run away
done
clear
B)
Eye closed when anything enter into it
done
clear
C)
Hand took up when piercing with needle
done
clear
D)
Digestive food goes forward in alimentary canal
done
clear
E)
None of the above
done
clear
View Answer play_arrow
question_answer 133) Colchicine prevents the mitosis of cells at which of the following stage?
A)
Anaphase
done
clear
B)
Metaphase
done
clear
C)
Prophase
done
clear
D)
Interphase
done
clear
E)
Telophase
done
clear
View Answer play_arrow
question_answer 134) Hydrolytic enzymes are found in
A)
lysosomes
done
clear
B)
peroxisomes
done
clear
C)
lomasomes
done
clear
D)
ribosomes
done
clear
E)
centrosome
done
clear
View Answer play_arrow
question_answer 135) The binomial names was accepted by all after the publication of the work by
A)
Linnaeus
done
clear
B)
Hooke
done
clear
C)
Bentham
done
clear
D)
Darwin
done
clear
E)
Lamarck
done
clear
View Answer play_arrow
question_answer 136) The pair of disease caused by virus is
A)
typhoid, tetanus
done
clear
B)
rabies, mumps
done
clear
C)
cholera, tuberculosis
done
clear
D)
AIDS, syphilis
done
clear
E)
TB, typhoid
done
clear
View Answer play_arrow
question_answer 137) An angiospermic leaf carries 16 chromosomes. The number of chromosomes in its endosperm will be
A)
16
done
clear
B)
24
done
clear
C)
12
done
clear
D)
8
done
clear
E)
32
done
clear
View Answer play_arrow
question_answer 138) A chemical fertilizin is produced form
A)
polar bodies
done
clear
B)
middle piece of sperm
done
clear
C)
acrosome
done
clear
D)
mature eggs
done
clear
E)
Sertoli cells
done
clear
View Answer play_arrow
question_answer 139) Free living, aerobic, non-photosynthetic nitrogen fixing bacterium is
A)
Azotobacter
done
clear
B)
E. coli
done
clear
C)
Nostoc
done
clear
D)
Salmonella
done
clear
E)
Clostridium
done
clear
View Answer play_arrow
question_answer 140) Which of the following hormones stimulates the stomach to secrete gastric juice?
A)
Gastrin
done
clear
B)
Enterokinase
done
clear
C)
Remin
done
clear
D)
Zymase
done
clear
E)
Secretin
done
clear
View Answer play_arrow
question_answer 141) The meristem responsible for extra stellar secondary growth in dicot stem is
A)
interfascicular cambium
done
clear
B)
intrafascicular cambium
done
clear
C)
intercalary meristem
done
clear
D)
phellogen
done
clear
E)
phelloderm
done
clear
View Answer play_arrow
question_answer 142) Minamata disease prevalent in industrial area due to pollution is due to
A)
lead
done
clear
B)
cadmium
done
clear
C)
mercury
done
clear
D)
zinc
done
clear
E)
arsenic
done
clear
View Answer play_arrow
question_answer 143) As a fungus completes its life cycle on two hosts it is termed as
A)
heteroecious
done
clear
B)
autoecious
done
clear
C)
heterothallic
done
clear
D)
monothallic
done
clear
E)
None of these
done
clear
View Answer play_arrow
question_answer 144) In an ovule the wall curvature is more pronounced and embryo sac become horse shoe shaped. The ovule is
A)
campylotropous
done
clear
B)
anatropous
done
clear
C)
amphitropous
done
clear
D)
orthotropous
done
clear
E)
hemianatropus
done
clear
View Answer play_arrow
question_answer 145) The stomata in CAM plants open during
A)
day
done
clear
B)
night
done
clear
C)
Both and
done
clear
D)
always closed
done
clear
E)
always open
done
clear
View Answer play_arrow
question_answer 146) Engler and Pranti published a phylogenetic system in the monograph
A)
Die Naturlichen pflanzen Familien
done
clear
B)
Historia Plantarum
done
clear
C)
Species Plantarum
done
clear
D)
Genera Plantarum
done
clear
E)
Origin of Species
done
clear
View Answer play_arrow
question_answer 147) Vascular bundles are arranged in a ring in the members of family
A)
Orchidaceae
done
clear
B)
Iridaceae
done
clear
C)
Euphorbiaceae
done
clear
D)
Lilliaceae
done
clear
E)
Palmae
done
clear
View Answer play_arrow
question_answer 148) The cloves which are used in food preparation are
A)
seeds
done
clear
B)
leaves
done
clear
C)
flower buds
done
clear
D)
stem tips
done
clear
E)
terminal buds
done
clear
View Answer play_arrow
question_answer 149) How many female flowers occur in a cyatbium?
A)
One
done
clear
B)
Two
done
clear
C)
Three
done
clear
D)
Four
done
clear
E)
Many
done
clear
View Answer play_arrow
question_answer 150) Which of the following features is absent in the family-Asteraceae?
A)
Cypsela fruit
done
clear
B)
Capirulum inflorescence
done
clear
C)
Hypogynous flowers
done
clear
D)
Syngenesious anthers
done
clear
E)
Pappus calyx
done
clear
View Answer play_arrow
question_answer 151) Casparian strip is made up of
A)
lignin
done
clear
B)
pectin
done
clear
C)
suberin
done
clear
D)
cellulose
done
clear
E)
starch
done
clear
View Answer play_arrow
question_answer 152) The phloem of angiosperms differs from that of other vascular plants by the presence of
A)
vessels
done
clear
B)
companion cells
done
clear
C)
tylosoides
done
clear
D)
albuminous cells
done
clear
E)
secretory cells
done
clear
View Answer play_arrow
question_answer 153) Fat is stored in the plant cell in
A)
lysosome
done
clear
B)
sphaerosome
done
clear
C)
microsome
done
clear
D)
peroxisome
done
clear
E)
macrophage
done
clear
View Answer play_arrow
question_answer 154) Which of the following is the unit of measurement of water potential?
A)
Watts
done
clear
B)
Joule
done
clear
C)
Pascal
done
clear
D)
Litre
done
clear
E)
Cubic centimeter
done
clear
View Answer play_arrow
question_answer 155) The number of NADPH molecules that are used during the conversion of carbon dioxide info one molecule of glucose
A)
1
done
clear
B)
4
done
clear
C)
6
done
clear
D)
8
done
clear
E)
12
done
clear
View Answer play_arrow
question_answer 156) DNA duplication takes place during
A)
cell division phase
done
clear
B)
entire interphase
done
clear
C)
only in \[{{G}_{1}}\]-phase
done
clear
D)
only in \[{{G}_{2}}\]-phase
done
clear
E)
only in S-phase
done
clear
View Answer play_arrow
question_answer 157) If RQ is 0.6 in a respiratory matabolism. It would mean that
A)
carbohydrates are used as respiratory, substrate
done
clear
B)
organic acids are used as respiratory substrate
done
clear
C)
the oxidation of the respiratory substrate consumed more oxygen than the amount of\[C{{O}_{2}}\] released
done
clear
D)
the oxidation of the respiratory substrate consumed more oxygen than the amount of \[C{{O}_{2}}\]released
done
clear
E)
the reaction is anaerobic
done
clear
View Answer play_arrow
question_answer 158) Which of the following gene clusters in bacteria is responsible for nitrogen fixation?
A)
Nod, nif, fix
done
clear
B)
Nod, ndf, nfx
done
clear
C)
Nod, nix, nfx
done
clear
D)
Ndx, nif, fix
done
clear
E)
Ndx, nif, nix
done
clear
View Answer play_arrow
question_answer 159) In Electron Transport System (ETS) which of the following cytochrome reacts with oxygen?
A)
\[Cyt.\text{-}b\]
done
clear
B)
\[Cyt.\text{-}{{a}_{3}}\]
done
clear
C)
\[Cyt.\text{-}{{b}_{6}}\]
done
clear
D)
\[Cyt.\text{-}f\]
done
clear
E)
\[Cyt.\text{-}{{b}_{3}}\]
done
clear
View Answer play_arrow
question_answer 160) The process in which haploid embryo is formed from haploid egg without fertilization is called
A)
apospory
done
clear
B)
agamospermy
done
clear
C)
apogamy
done
clear
D)
vegetative reproduction
done
clear
E)
adventive polyembryony
done
clear
View Answer play_arrow
question_answer 161) \[C{{O}_{2}},\,C{{H}_{4}},\,{{N}_{2}}O\] and \[CFCs\]are called green house gases because they can absorb
A)
ultraviolet radiation
done
clear
B)
long wave infra red radiation
done
clear
C)
visible light radiation
done
clear
D)
\[X\text{-}\]rays radiation
done
clear
E)
\[\gamma \text{-}\]rays radiation
done
clear
View Answer play_arrow
question_answer 162) The transition zone between two communities is known as
A)
ecotone
done
clear
B)
keystone
done
clear
C)
edge effect
done
clear
D)
critical link species
done
clear
E)
edge species
done
clear
View Answer play_arrow
question_answer 163) Which of the following statements is false?
A)
Male round worm is smaller than female
done
clear
B)
Earthworms are hermaphrodites
done
clear
C)
Echinoderms are protostomous coelomates
done
clear
D)
Human teeth are anatomically comparable to scales of shark
done
clear
E)
Hair is a derivative of skin
done
clear
View Answer play_arrow
question_answer 164) Triploblastic, unsegmented, acoelomate exhibiting bilateral symmetry and reproducing both asexually and sexually, with some parasitic forms. The above description is characteristic of the phylum
A)
Annelida
done
clear
B)
Ctenophora
done
clear
C)
Cnidaria
done
clear
D)
Porifera
done
clear
E)
Platyhelminthes
done
clear
View Answer play_arrow
question_answer 165) Dr. Khorana and his collegues synthesized an RNA molecule with repeating sequence of UG n bases (UG UG UG UG UG UG). It produced a tetrapeptide with alternating sequence of cystein and valine. It proves that codons for cystein and valine is
A)
UGU and GUU
done
clear
B)
UGU and GUG
done
clear
C)
UUG and GGU
done
clear
D)
GUG and UGU
done
clear
E)
GUU and UGU
done
clear
View Answer play_arrow
question_answer 166) Inter-vertebral disc consists of a shock absorber connective tissue known as
A)
hyaline cartilage
done
clear
B)
elastic cartilage
done
clear
C)
fibro cartilage
done
clear
D)
recticulo cartilage
done
clear
E)
calacified cartilage
done
clear
View Answer play_arrow
question_answer 167) Blackening of urine when exposed to air is a metabolic disorder in human beings. This is due to
A)
phenylalanine
done
clear
B)
tyrosine
done
clear
C)
valine replacing glutamine
done
clear
D)
glutamine replacing valine
done
clear
E)
homogentisic acid
done
clear
View Answer play_arrow
question_answer 168) Phenylketonuria is a genetic disorder of
A)
trisomic condition
done
clear
B)
mpnosomic condition
done
clear
C)
autosomal dominant gene
done
clear
D)
autosomal recessive gene
done
clear
E)
X-linked
done
clear
View Answer play_arrow
question_answer 169) Neoteny refers to
A)
development of gonads
done
clear
B)
pre-adult animal
done
clear
C)
metamorphosis
done
clear
D)
retention of larval or embryonic trait in the adult body
done
clear
E)
precociocus development
done
clear
View Answer play_arrow
question_answer 170) In earthworm the dorsal wall of the intestine from the 26th segment to 95th segment forms a median internal fold called
A)
trochophore
done
clear
B)
typhlosole
done
clear
C)
clitellum
done
clear
D)
trachea
done
clear
E)
nephridium
done
clear
View Answer play_arrow
question_answer 171) Reproductive isolation means
A)
inability to interbreed
done
clear
B)
breed in isolation
done
clear
C)
ability to interbreed
done
clear
D)
breeding in a species
done
clear
E)
None of the above
done
clear
View Answer play_arrow
question_answer 172) The plant which gives agar-agar is
A)
Laminaria
done
clear
B)
Chara
done
clear
C)
Sargassum
done
clear
D)
Gelidium
done
clear
E)
Cephaleuros
done
clear
View Answer play_arrow
question_answer 173) Rhythmic heart beat is maintained by a highly specialized excitatory and conductive system. The correct sequence of events will be?
A)
Atrio-ventricular node-bundle of His-Sino-atrio node-network of Purkinje fibres
done
clear
B)
Network of Purkinje fibres-Atrio-ventricular node-Sino-Atrio node-bundle of His
done
clear
C)
Atrio-ventricular node-Sino-Atrio node-bundle of His-Purkinje fibres
done
clear
D)
Sino-atrial node-Atrio-ventricular node- bundle of His-Purkinje fibres
done
clear
E)
Sino-atrial node-Atrio-ventricular node- Purkinje fibres-bundle of His
done
clear
View Answer play_arrow
question_answer 174) Okazaki fragments are joined in a correct sequence by
A)
DNA polymerase
done
clear
B)
DNA ligase
done
clear
C)
RNA polymerase
done
clear
D)
primase
done
clear
E)
helicase
done
clear
View Answer play_arrow
question_answer 175) The muscle band that remains unchanged during contraction and relaxation of the skeletal muscle is
A)
1
done
clear
B)
H
done
clear
C)
A
done
clear
D)
A line
done
clear
E)
H and Z line
done
clear
View Answer play_arrow
question_answer 176) Hypothalamus does not control
A)
hunger and satiety
done
clear
B)
thermo regulation
done
clear
C)
ibido
done
clear
D)
creative thinking and consciousness
done
clear
E)
osomo regulation
done
clear
View Answer play_arrow
question_answer 177) A gland which gradually atrophies at the age of 14-16 due to the activities of sex gland is
A)
thyroid
done
clear
B)
parathyroid
done
clear
C)
pancreas
done
clear
D)
pineal
done
clear
E)
thymus
done
clear
View Answer play_arrow
question_answer 178) Rate of breathing is controlled by
A)
the amount of freely available oxygen
done
clear
B)
carbon dioxide
done
clear
C)
muscular functions of the body
done
clear
D)
stress
done
clear
E)
All of the above
done
clear
View Answer play_arrow
question_answer 179) You are watching a horror move and you notice your heart is beating fast and mouth is dry. It is because of
A)
fight and flight response
done
clear
B)
autonomic nervous system
done
clear
C)
sympathetic nervous system
done
clear
D)
para sympathetic nervous system
done
clear
E)
Both and
done
clear
View Answer play_arrow
question_answer 180) In males testes are contained in the scrotal sacs because
A)
other organs do not make space for the testes in the abdominal cavity
done
clear
B)
testes in the abdomen will hamper maturation of sperms
done
clear
C)
it provides temperature that is slightly lower than body temperature required for formation of functional sperms
done
clear
D)
it facilitates ejaculation
done
clear
E)
testes in the abdomen will accelerate maturation of sperms
done
clear
View Answer play_arrow
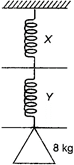
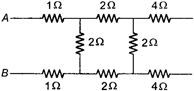
 is an example of
is an example of
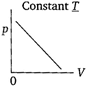
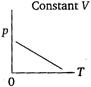
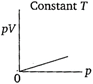
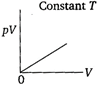
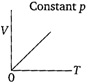
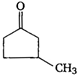
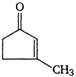
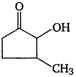
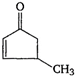
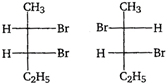 They are
They are
