question_answer 1) If the internal energy of\[{{n}_{1}}\]moles of Heat temperature 10 T is equal to the internal energy of\[{{n}_{2}}\] mole of hydrogen at temperature 6T. The ratio of \[{{n}_{1}}\]/\[{{n}_{2}}\] is
A)
3/5
done
clear
B)
2
done
clear
C)
1
done
clear
D)
5/3
done
clear
View Answer play_arrow
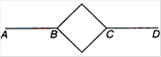
question_answer 2)
Six identical metallic rods are joined together in a pattern as shown in the figure. Points A and D are maintained at temperatures 60°C and 240°C. The temperature of the junction B will be
A)
\[120{}^\circ C\]
done
clear
B)
\[150{}^\circ C\]
done
clear
C)
\[60{}^\circ C~~\]
done
clear
D)
\[80{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 3) The motion of a particle executing SHM in one dimension is described by x = - 0.3 sin\[(t+\pi /4),\]where x is in metre and \[t\]in second. The frequency of oscillation in Hz is
A)
\[3\]
done
clear
B)
\[1/2\pi \]
done
clear
C)
\[\pi /2\]
done
clear
D)
\[1/\pi \]
done
clear
View Answer play_arrow
question_answer 4) \[At\,t=0,\] a stone of mass 10 g is thrown straight up from the ground level with a speed 10 m/s. After 1 s a second stone of the same mass is thrown from the same position with a speed 20 m/s. What is the position of the 1st stone from the ground level at that moment? (take g =10 m/s2)
A)
10 m
done
clear
B)
1 m
done
clear
C)
2m
done
clear
D)
5 m
done
clear
View Answer play_arrow
question_answer 5) Two stars of masses m, and m2 are part of a binary star system. The radii of their orbits are \[{{r}_{1}}\]and \[{{r}_{2}}\] respectively, measured from the CM of the system. The magnitude of acceleration of \[{{m}_{1}}\] is
A)
\[\frac{{{m}_{1}}{{m}_{2}}G}{{{({{r}_{1}}+{{r}_{2}})}^{2}}}\]
done
clear
B)
\[\frac{{{m}_{1}}G}{{{({{r}_{1}}+{{r}_{2}})}^{2}}}\]
done
clear
C)
\[\frac{{{m}_{2}}G}{{{({{r}_{1}}+{{r}_{2}})}^{2}}}\]
done
clear
D)
\[\frac{{{m}_{1}}+{{m}_{2}}}{{{({{r}_{1}}+{{r}_{2}})}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 6) A pendulum is made to hang from the ceiling of an elevator. It has period of T sec [for small angles). The elevator is made to accelerate upwards with\[10\text{ }m/{{s}^{2}}\]. The period of the pendulum now will be (Take g \[=10\text{ }m/{{s}^{2}}\])
A)
\[T\sqrt{2}\]
done
clear
B)
infinite
done
clear
C)
\[T/\sqrt{2}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 7) A person is measuring his weight by standing on a weighing machine inside a lift. When the lift is at rest, the machine shows his weight to be 55 kg. In between the floor when the lift is moving up with a constant speed of 10 km/h, he again measures his weight, which is
A)
55 kg
done
clear
B)
65kg
done
clear
C)
50 kg
done
clear
D)
45 kg
done
clear
View Answer play_arrow
question_answer 8) A child travelling in a train throws a ball outside with a speed v. According to a child who is standing on the ground, the speed of the ball is
A)
same as v
done
clear
B)
greater than v
done
clear
C)
less than v
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 9) A body of mass M at rest explodes into three pieces, two of them of mass M/4 each, are thrown off in perpendicular directions with velocities of 3 m/s and 4 m/s respectively. The third piece will be thrown off with a velocity of
A)
1.5 m/s
done
clear
B)
2 m/s
done
clear
C)
2.5 m/s
done
clear
D)
3 m/s
done
clear
View Answer play_arrow
question_answer 10) A tunnel has been dug through the centre of the earth and a ball is released in it. It executes SHM with time period
A)
42 min
done
clear
B)
1 day
done
clear
C)
1 h
done
clear
D)
84.6 min
done
clear
View Answer play_arrow
question_answer 11) An ideal spring with spring constant k = 200 N/m is fixed on one end of a wall. If the spring is pulled with a force 10 N at the other end along its length, how much it will be extended?
A)
5 cm
done
clear
B)
2m
done
clear
C)
2 cm
done
clear
D)
5 m
done
clear
View Answer play_arrow
question_answer 12) An iron block of mass 5 kg is kept on a trolley. If the trolley is being pushed with an acceleration of 5 m/s2, what will be the force of friction between the block and the trolley surface? (Take the coefficient of static friction between the block and the trolley surface to be 0,8).
A)
Zero
done
clear
B)
5 N
done
clear
C)
4 N
done
clear
D)
25 N
done
clear
View Answer play_arrow
question_answer 13) A ball with charge 50 e is placed at the centre of a hollow spherical shell which has a net charge of - 50 e. What is the charge on the shells outer surface?
A)
-50e
done
clear
B)
Zero
done
clear
C)
-100e
done
clear
D)
+100e
done
clear
View Answer play_arrow
question_answer 14) If the charge on a capacitor is doubled, the value of its capacitance C will be
A)
doubled
done
clear
B)
halved
done
clear
C)
remain the same
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 15) A point mass is placed inside a thin spherical shell of radius \[H\] and mass \[M\] at a distance R/2 from the centre of the shell. The gravitational force exerted by the shell on the point mass is.
A)
\[\frac{GM}{2{{R}^{2}}}\]
done
clear
B)
\[\frac{GM}{2{{R}^{2}}}\]
done
clear
C)
zero
done
clear
D)
\[\frac{GM}{4{{R}^{2}}}\]
done
clear
View Answer play_arrow
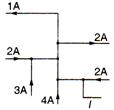
question_answer 16)
The magnitude and direction of current \[I\] (in A) Indicated in the following circuit is
A)
\[14\to \]
done
clear
B)
\[8\to \]
done
clear
C)
\[\leftarrow 4\]
done
clear
D)
\[\leftarrow 8\]
done
clear
View Answer play_arrow
question_answer 17) An electric motor operating on 15V supply draws a current of 5 A and yields mechanical power of 60 W. The energy lost as heat in one hour (in kJ) is
A)
0.54
done
clear
B)
5.4
done
clear
C)
54
done
clear
D)
540
done
clear
View Answer play_arrow
question_answer 18) The frequency of cyclotron motion of a charged particle in a magnetic field is dependent of its
A)
charge e
done
clear
B)
mass m
done
clear
C)
velocity
done
clear
D)
elm ratio
done
clear
View Answer play_arrow
question_answer 19) Iron would become paramagnetic at about
A)
\[200{}^\circ C\]
done
clear
B)
\[400{}^\circ C\]
done
clear
C)
\[600{}^\circ C\]
done
clear
D)
\[800{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 20) The electric dipole moment of an electron and a proton 4.3 nm apart is
A)
\[6.88\times {{10}^{-28}}\,\,Cm\]
done
clear
B)
\[2.56\times {{10}^{-29}}{{C}^{2}}/m\]
done
clear
C)
\[3.72\times {{10}^{-14}}\,\,C/m\]
done
clear
D)
\[1.1\times {{10}^{-46}}\,\,{{C}^{2}}m\]
done
clear
View Answer play_arrow
question_answer 21) A parallel plate capacitor of a capacitance of 1 F would have the plate area of about
A)
\[100\text{ }{{m}^{2}}\]
done
clear
B)
\[1\text{ }k{{m}^{2}}\]
done
clear
C)
\[100\text{ }k{{m}^{2}}\]
done
clear
D)
\[1000\text{ }k{{m}^{2}}\]
done
clear
View Answer play_arrow
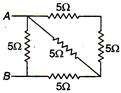
question_answer 22)
The equivalent resistance between the points A and B in the following circuit is
A)
3.120
done
clear
B)
1.560
done
clear
C)
6.24 0
done
clear
D)
12.48 P
done
clear
View Answer play_arrow
question_answer 23) A galvanometer coil has a resistance of 10 \[\Omega \]and the ammeter shows full scale deflection for a current of 1mA. The shunt resistance required to convert the galvanometer into an ammeter of range 0-100mA is about
A)
10\[\Omega \]
done
clear
B)
1\[\Omega \]
done
clear
C)
0.1\[\Omega \]
done
clear
D)
12.48\[\Omega \]
done
clear
View Answer play_arrow
question_answer 24) A thermo-emf V appears across a conductor maintained at a temperature difference T. The Thomson coefficient is then given by
A)
\[-T\frac{{{d}^{2}}V}{d{{T}^{2}}}\]
done
clear
B)
\[{{T}^{2}}\frac{dV}{dT}\]
done
clear
C)
\[-\frac{1}{T}\frac{{{d}^{2}}V}{d{{T}^{2}}}\]
done
clear
D)
\[-\frac{1}{{{T}^{2}}}\frac{dV}{dT}\]
done
clear
View Answer play_arrow
question_answer 25) How much current should be passed through a silver voltmeter to deposit 200 g of silver per hour on the cathode? (Faraday constant = 96500 C/mol and relative atomic mass of silver is 108)
A)
50mA
done
clear
B)
50 A
done
clear
C)
15mA
done
clear
D)
15 A
done
clear
View Answer play_arrow
question_answer 26) The relative magnetic permeability of ferromagnetic materials is of the order of
A)
10
done
clear
B)
100
done
clear
C)
1000
done
clear
D)
10000
done
clear
View Answer play_arrow
question_answer 27) A solenoid is placed inside another solenoid, the lengths of both being equal carrying same magnitude of current. The other parameters like radius and number of turns are in the ratio 1:2 for the two solenoids. The mutual inductance on each other would be
A)
\[{{M}_{12}}={{M}_{21}}\]
done
clear
B)
\[{{M}_{12}}=2{{M}_{21}}\]
done
clear
C)
\[2{{M}_{12}}=2{{M}_{21}}\]
done
clear
D)
\[{{M}_{12}}=4{{M}_{21}}\]
done
clear
View Answer play_arrow
question_answer 28) The average magnetic energy density of an electromagnetic wave of wavelength\[\lambda \] travelling in free space is given by
A)
\[\frac{{{B}^{2}}}{2\lambda }\]
done
clear
B)
\[\frac{{{B}^{2}}}{2{{\mu }_{o}}}\]
done
clear
C)
\[\frac{2{{B}^{2}}}{{{\mu }_{o}}\lambda }\]
done
clear
D)
\[\frac{B}{{{\mu }_{o}}\lambda }\]
done
clear
View Answer play_arrow
question_answer 29) The numerical aperture for a human eye is of the order of
A)
1
done
clear
B)
0.1
done
clear
C)
0.01
done
clear
D)
0.001
done
clear
View Answer play_arrow
question_answer 30) The radius of a copper nucleus is of the order of
A)
\[{{10}^{-16}}\,m\]
done
clear
B)
\[{{10}^{-14}}\,m\]
done
clear
C)
\[{{10}^{-12}}\,m\]
done
clear
D)
\[{{10}^{-9}}\,m\]
done
clear
View Answer play_arrow
question_answer 31) A material has N atoms in its crystal structure which is a hexagonal close packed. Then the number of electron states in a band is
A)
N
done
clear
B)
2N
done
clear
C)
4N
done
clear
D)
6N
done
clear
View Answer play_arrow
question_answer 32) If \[{{10}^{10}}\] electrons are acquired by a body every second, the time required for the body to get a total charge of 1 C will be
A)
2h
done
clear
B)
2 days
done
clear
C)
2yr
done
clear
D)
20 yr
done
clear
View Answer play_arrow
question_answer 33) The magnetic field in a plane electromagnetic wave is given by \[{{B}_{y}}=2\times {{10}^{-7}}\] \[\sin \,(0.5\times {{10}^{-3}}\times +1.5\times {{10}^{11}}t).\] This electromagnetic wave is
A)
a visible light
done
clear
B)
an infrared wave
done
clear
C)
a microwave
done
clear
D)
a radio wave
done
clear
View Answer play_arrow
question_answer 34) Which of these particles (having the same kinetic energy) has the shortest de-Broglie wave length?
A)
Electron
done
clear
B)
Alpha particle
done
clear
C)
Proton
done
clear
D)
Neutron
done
clear
View Answer play_arrow
question_answer 35) A radioactive isotope has a half-life of T yr. How long will it take the activity to reduce to 1% of its original value?
A)
3.2 Tyr
done
clear
B)
4.6 Tyr
done
clear
C)
6.6 Tyr
done
clear
D)
9.2 Tyr
done
clear
View Answer play_arrow
question_answer 36) Although carbon, silicon and germanium have same lattice structure and four valence electrons each, their band structure leads to the energy gaps as
A)
\[{{E}_{g}}(Si)<{{E}_{g}}(Ge)<{{E}_{g}}(C)\]
done
clear
B)
\[{{E}_{g}}(Si)>{{E}_{g}}(Ge)<{{E}_{g}}(C)\]
done
clear
C)
\[{{E}_{g}}(Si)<{{E}_{g}}(Ge)>{{E}_{g}}(C)\]
done
clear
D)
\[{{E}_{g}}(Si)>{{E}_{g}}(Ge)>{{E}_{g}}(C)\]
done
clear
View Answer play_arrow
question_answer 37) When a lens of refractive index \[{{n}_{1}}\] is placed in a liquid of refractive index \[{{n}_{2}}\] the lens looks to be disappeared only if
A)
\[{{n}_{1}}={{n}_{2}}/2\]
done
clear
B)
\[{{n}_{1}}=3{{n}_{2}}/4\]
done
clear
C)
\[{{n}_{1}}={{n}_{2}}\]
done
clear
D)
\[{{n}_{1}}=5{{n}_{2}}/4\]
done
clear
View Answer play_arrow
question_answer 38) The density of a nucleus of mass number A is proportional to
A)
\[{{A}^{3}}\]
done
clear
B)
\[{{A}^{1/3}}\]
done
clear
C)
\[{{A}^{1}}\]
done
clear
D)
\[A{}^\circ \]
done
clear
View Answer play_arrow
question_answer 39) A p-n-p transistor having AC current gain of 50 is used to make an amplifier of a voltage gain of 5. What will be the power gain of the amplifier?
A)
125
done
clear
B)
178
done
clear
C)
250
done
clear
D)
354
done
clear
View Answer play_arrow
question_answer 40) Which of the following frequencies will be suitable for beyond-the-horizon communication?
A)
10 kHz
done
clear
B)
10 MHz
done
clear
C)
1 GHz
done
clear
D)
1000 GHz
done
clear
View Answer play_arrow
question_answer 41) In an air collision between an aeroplane and a bird, the force experienced by the bird as compared to that of the aeroplane is
A)
very high
done
clear
B)
equal
done
clear
C)
less
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 42) An iron ball of mass M is hanged from the ceiling by a spring with a spring constant k. It executes a SHM with a period P. If the mass of the ball is increased by four times, the new period will be
A)
4P
done
clear
B)
P/4
done
clear
C)
2P
done
clear
D)
P
done
clear
View Answer play_arrow
question_answer 43) Two stones of equal masses are dropped from a rooftop of height h one after another. Their separation distance against time will
A)
remain the same
done
clear
B)
increase
done
clear
C)
decrease
done
clear
D)
be zero
done
clear
View Answer play_arrow
question_answer 44) The angle subtended by a circular disk of diameter 2 cm at a distance 1000 cm from your eye is
A)
\[0.2{}^\circ \]
done
clear
B)
\[0.002{}^\circ \]
done
clear
C)
\[0.11{}^\circ \]
done
clear
D)
\[0.22{}^\circ \]
done
clear
View Answer play_arrow
question_answer 45) The mass and radius of the sun are \[1.99\times {{10}^{30}}kg\] and \[R=6.96\times 10{}^\circ m\]. The escape velocity of a rocket from the sun is
A)
\[11.2km/s\]
done
clear
B)
\[2.38\text{ }km/s\]
done
clear
C)
\[59.5\text{ }km/s\]
done
clear
D)
\[618\text{ }km/s\]
done
clear
View Answer play_arrow
question_answer 46) A satellite of mass m is orbiting close to the surface of the earth (Radius\[R=6400\text{ }km\]) has a kinetic energy K. The corresponding kinetic energy of the satellite to escape from the earths gravitational field is
A)
K
done
clear
B)
2K
done
clear
C)
mg R
done
clear
D)
mK
done
clear
View Answer play_arrow
question_answer 47) The height from the earth surface at which the value of acceleration due to gravity reduces to 1/4th of its value at earths surface (assume earth to be sphere of radius 6400 km)
A)
6400 km
done
clear
B)
2400 km
done
clear
C)
2946 km
done
clear
D)
1600 km
done
clear
View Answer play_arrow
question_answer 48) 10 moles of an ideal monoatomic gas at \[10{}^\circ C\] is mixed with 20 moles of another monoatomic gas at\[20{}^\circ C\]. Then the temperature of the mixture is
A)
\[15.5{}^\circ C\]
done
clear
B)
\[15{}^\circ C\]
done
clear
C)
\[16{}^\circ C\]
done
clear
D)
\[16.6{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 49) An ideal gas is made to go through a cyclic thermodynamical process in four steps. The amounts of heat involved are\[{{Q}_{1}}=100\,J,\] \[{{Q}_{2}}=300\,J,\] \[{{Q}_{2}}=400\,J,\] and \[{{Q}_{4}}=100\,J,\] respectively. The corresponding work involved are\[{{W}_{1}}=300\,J,\] \[{{W}_{2}}=-100\,J,\] \[{{W}_{3}}=400\,J\]and \[{{W}_{4.}}\] What is the value of\[{{W}_{4.}}\]?
A)
100 J
done
clear
B)
- 100 J
done
clear
C)
500 J
done
clear
D)
- 700 J
done
clear
View Answer play_arrow
question_answer 50) Two cylinders of equal sizes are filled with equal amounts of ideal diatomic gas at room temperature. Both the cylinders are fitted with pistons. In cylinder A, the piston is free to move, while in cylinder B the piston is fixed. When same amount of heat is supplied to both the cylinders, the temperature of the gas in cylinder A raises by 30K. What will be the rise in temperature of the gas in cylinder B?
A)
42 K
done
clear
B)
30 K
done
clear
C)
20 K
done
clear
D)
56 K
done
clear
View Answer play_arrow
question_answer 51) Which one of the following is the correct increasing order of the magnitude of ionic radii of \[C{{e}^{3+}},L{{a}^{3+}},P{{m}^{3+}}\] and\[Y{{b}^{3+}}\]?
A)
\[Y{{b}^{3+}}<P{{m}^{3+}}<L{{a}^{3+}}<C{{e}^{3+}}\]?
done
clear
B)
\[Y{{b}^{3+}}<P{{m}^{3+}}<C{{e}^{3+}}<L{{a}^{3+}}\]
done
clear
C)
\[P{{m}^{3+}}<L{{a}^{3+}}<C{{e}^{3+}}<Y{{b}^{3+}}\]
done
clear
D)
\[C{{e}^{3+}}<Y{{b}^{3+}}<P{{m}^{3+}}<L{{a}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 52) The number of unpaired electrons in tetrahedral \[[Ni{{(CO)}_{4}}]\] is
A)
0
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 53) Choose the correct order regarding the bond order.
A)
\[O_{2}^{2-}>O_{2}^{+}>O_{2}^{-}>{{O}_{2}}\]
done
clear
B)
\[O_{2}^{+}>O_{2}^{2-}>O_{2}^{-}>{{O}_{2}}\]
done
clear
C)
\[O_{2}^{+}>{{O}_{2}}>O_{2}^{-}>O_{2}^{2-}\]
done
clear
D)
\[{{O}_{2}}>O_{2}^{-}>O_{2}^{2-}>O_{2}^{+}\]
done
clear
View Answer play_arrow
question_answer 54) Which one of the following metal ions is essential inside the cell for the metabolism of glucose/synthesis of proteins?
A)
\[C{{a}^{2+}}\]
done
clear
B)
\[M{{g}^{2+}}\]
done
clear
C)
\[N{{a}^{+}}\]
done
clear
D)
\[{{K}^{+}}\]
done
clear
View Answer play_arrow
question_answer 55) Which noble gas is most abundant in atmosphere?
A)
He
done
clear
B)
Ne
done
clear
C)
Ar
done
clear
D)
Kr
done
clear
View Answer play_arrow
question_answer 56) On \[\alpha \]-decay \[_{92}^{238}U\] produces
A)
\[_{93}^{238}Np\]
done
clear
B)
\[_{90}^{234}Th\]
done
clear
C)
\[_{91}^{234}Pa\]
done
clear
D)
\[_{92}^{234}U\]
done
clear
View Answer play_arrow
question_answer 57) Which of the following methods is used for obtaining aluminium metal?
A)
Electrolysing fused \[A{{l}_{2}}{{O}_{3}}\] and cryolite
done
clear
B)
By heating \[A{{l}_{2}}{{O}_{3}}\] with carbon
done
clear
C)
By heating \[A{{l}_{2}}{{O}_{3}}\] in Muffle furnace
done
clear
D)
By a process called pyrometallurgy
done
clear
View Answer play_arrow
question_answer 58) A hydroxy acid on heating gives a 5-membered lactone. The acid is
A)
\[C{{H}_{2}}OHC{{H}_{2}}C{{H}_{2}}COOH\]
done
clear
B)
\[C{{H}_{3}}CHOHC{{H}_{2}}COOH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CHOHCOOH\]
done
clear
D)
\[C{{H}_{3}}CHOHCHOHCOOH\]
done
clear
View Answer play_arrow
question_answer 59) Which of the following statements is not correct?
A)
Caprolactam is the monomer of nylon-6
done
clear
B)
Terylene is a polyester polymer
done
clear
C)
Phenol formaldehyde resin is known as bakelite
done
clear
D)
The monomer of natural rubber is butadiene
done
clear
View Answer play_arrow
question_answer 60) The anticodon of transfer RNA for the messenger RNA codon G-C-A is
A)
T-G-A
done
clear
B)
G-U-T
done
clear
C)
A-G-T
done
clear
D)
C-G-U
done
clear
View Answer play_arrow
question_answer 61) The secondary structure of proteins is derived from
A)
peptide linkages
done
clear
B)
hydrogen bonding
done
clear
C)
disulphide linkages
done
clear
D)
folding of chains
done
clear
View Answer play_arrow
question_answer 62) The source of energy in a cellular reaction is
A)
chemical energy
done
clear
B)
light energy
done
clear
C)
heat energy
done
clear
D)
solar radiation
done
clear
View Answer play_arrow
question_answer 63) The final product in the following Jaction sequence is p-chloroamime \[\xrightarrow[{{O}^{o}}C-{{5}^{o}}C]{NaN{{O}_{2}},\,HCl}?\xrightarrow{KCN}?\xrightarrow{LiAl{{H}_{4}}}\]?
A)
p -chlorobenzamide
done
clear
B)
p -chlorophenol
done
clear
C)
p -chlorobenzylamine
done
clear
D)
p -chlorobenzylalcohol
done
clear
View Answer play_arrow
question_answer 64) Which of the following is not a biliquid propellant?
A)
\[{{N}_{2}}{{O}_{4}}+\] unsymmetrical dimethyl hydrazine
done
clear
B)
Nitroglycerine + nitrocellulose
done
clear
C)
Hydrazine \[+{{N}_{2}}{{O}_{4}}\]
done
clear
D)
Kerosene oil + liquid oxygen
done
clear
View Answer play_arrow
question_answer 65) The dyes which are used in reduced state and are then oxidised in the fabric by air are called
A)
azo dyes
done
clear
B)
dispersed dyes
done
clear
C)
basic dyes
done
clear
D)
vat dyes
done
clear
View Answer play_arrow
question_answer 66) Which of the following compounds will not give Lassaignes test for nitrogen?
A)
\[N{{H}_{2}}N{{H}_{2}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}NHN{{H}_{2}}\]
done
clear
C)
\[PhN=NPh\]
done
clear
D)
\[N{{H}_{2}}CON{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 67) The compound with molecular formula \[{{C}_{8}}{{H}_{10}}\] which will give two isomers on electrophilic substitution with \[C{{l}_{2}}/FeC{{l}_{3}}\]or \[HN{{O}_{3}}/{{H}_{2}}S{{O}_{4}}\] is
A)
p-dimethyl benzene
done
clear
B)
m-dimethyl benzene
done
clear
C)
o-dimethyl benzene
done
clear
D)
ethyl benzene
done
clear
View Answer play_arrow
question_answer 68) Decreasing order of reactivity in Williamson ether synthesis of the following is (I) \[M{{e}_{3}}CC{{H}_{2}}Br\], (II) \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Br\], (III) \[C{{H}_{2}}=CHC{{H}_{2}}Cl\], (IV) \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Cl\]
A)
\[III>II>IV>I\]
done
clear
B)
\[I>II>IV>III\]
done
clear
C)
\[II>III>IV>I\]
done
clear
D)
\[I>III>II>IV\]
done
clear
View Answer play_arrow
question_answer 69) Identify the product in the reaction \[PhC\equiv CMe\xrightarrow{{{H}_{3}}{{O}^{+}},H{{g}^{2+}}}\]?
A)
\[phC{{H}_{2}}C{{H}_{2}}CHO\]
done
clear
B)
\[phCOC{{H}_{2}}C{{H}_{3}}\]
done
clear
C)
\[phC{{H}_{2}}COC{{H}_{3}}\]
done
clear
D)
\[phCOCOMe\]
done
clear
View Answer play_arrow
question_answer 70) The order of rate of hydrolysis of alkyl halides \[{{1}^{o}},{{2}^{o}},{{3}^{o}}\] and \[C{{H}_{3}}X\] by the \[{{S}_{N}}2\] pathway is
A)
\[{{1}^{o}}>{{2}^{o}}>{{3}^{o}}>C{{H}_{3}}X\]
done
clear
B)
\[C{{H}_{3}}X>{{3}^{o}}>{{2}^{o}}>{{1}^{o}}\]
done
clear
C)
\[C{{H}_{3}}X>{{1}^{o}}>{{2}^{o}}>{{3}^{o}}\]
done
clear
D)
\[{{3}^{o}}>{{2}^{o}}>{{1}^{o}}>C{{H}_{3}}X\]
done
clear
View Answer play_arrow
question_answer 71) Compound A undergoes formation of cyanohydrin which on hydrolysis gives lactic acid\[(C{{H}_{3}}CHOHCOOH)\]. Therefore, compound A is
A)
formaldehyde
done
clear
B)
acetaldehyde
done
clear
C)
acetone
done
clear
D)
benzaldehyde
done
clear
View Answer play_arrow
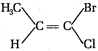
question_answer 72)
Which of the following is a pair of geometric isomers?
A)
(i) and (ii)
done
clear
B)
(i) and (in)
done
clear
C)
(i) and (iv)
done
clear
D)
(ii) and (iii)
done
clear
View Answer play_arrow
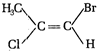
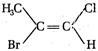
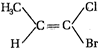
question_answer 73) How many chiral stereoisomers can be drawn for 2-bromo-3-chlorobutane?
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 74) Which of the following statements is not correct?
A)
Aldehydes and ketones undergo micleophilic additions
done
clear
B)
Aldehydes and ketones undergo olectrophilic substitutions
done
clear
C)
Aldehydes and betones contain polar carbonyl groups
done
clear
D)
Lower members of aldehydes and ketones are soluble in water due to hydrogen bonding
done
clear
View Answer play_arrow
question_answer 75) How many gram of ice at \[{{0}^{o}}C\] can be melted by the addition of 500 J of heat? (The molar heat of fusion for ice is 6.02 k] \[mo{{l}^{-1}}\])
A)
0.0831 g
done
clear
B)
1.50 g
done
clear
C)
3.01 g
done
clear
D)
12.0 g
done
clear
View Answer play_arrow
question_answer 76)
A 1.0 g sample of substance A at \[{{100}^{o}}C\] is added to 100 mL of \[{{H}_{2}}O\] at \[{{25}^{o}}C\]. Using separate 100 mL portions of \[{{H}_{2}}O\], the procedure is repeated with substance B and then with substance C. How will the final temperatures of the water compare? Substance Specific heat A \[0.60\,\,J{{g}^{-1}}\,{{\,}^{o}}{{C}^{-1}}\] B \[0.40\,\,J{{g}^{-1}}\,{{\,}^{o}}{{C}^{-1}}\] C \[0.20\,\,J{{g}^{-1}}\,{{\,}^{o}}{{C}^{-1}}\]
A)
\[{{T}_{C}}>{{T}_{B}}>{{T}_{A}}\]
done
clear
B)
\[{{T}_{B}}>{{T}_{A}}>{{T}_{C}}\]
done
clear
C)
\[{{T}_{A}}>{{T}_{B}}>{{T}_{C}}\]
done
clear
D)
\[{{T}_{A}}={{T}_{B}}={{T}_{C}}\]
done
clear
View Answer play_arrow
question_answer 77) By what factor does the average velocity of a gaseous molecule increase when the absolute temperature is doubled?
A)
1.4
done
clear
B)
2.0
done
clear
C)
2.8
done
clear
D)
4.0
done
clear
View Answer play_arrow
question_answer 78) When solid lead iodide is added to water, the equilibrium concentration of \[{{I}^{-}}\] becomes\[2.6\times {{10}^{-3}}M\]. What is the \[{{K}_{sp}}\] for \[Pb{{I}_{2}}\]?
A)
\[2.6\times {{10}^{-3}}M\]
done
clear
B)
\[8.8\times {{10}^{-9}}\]
done
clear
C)
\[1.8\times {{10}^{-8}}\]
done
clear
D)
\[3.5\times {{10}^{-8}}\]
done
clear
View Answer play_arrow
question_answer 79) Which values can be obtained from the information represented by the vapour pressure curve of a quid? [a] Normal boiling point [b] Normal freezing point [c] Enthalpy of vaporisation
A)
[A] only
done
clear
B)
[A] and [B] only
done
clear
C)
[A] and [C] only
done
clear
D)
[A], [B] and [C]
done
clear
View Answer play_arrow
question_answer 80) The free energy of formation of NO is 78 kJ \[mo{{l}^{-1}}\] at the temperature of an automobile engine (1000 K). What is the equilibrium constant for this reaction at 1000 K? \[\frac{1}{2}{{N}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)NO(g)\]
A)
\[8.4\times {{10}^{-5}}\]
done
clear
B)
\[7.1\times {{10}^{-9}}\]
done
clear
C)
\[4.2\times {{10}^{-10}}\]
done
clear
D)
\[1.7\times {{10}^{-19}}\]
done
clear
View Answer play_arrow
question_answer 81) The first-order reaction, \[2{{N}_{2}}O(g)\to 2{{N}_{2}}(g)+{{O}_{2}}(g)\] has a rate constant of \[1.3\times {{10}^{-11}}{{s}^{-1}}\] at \[{{270}^{o}}C\]and \[4.5\times {{10}^{-10}}{{s}^{-1}}\]at\[{{350}^{o}}C\]. What is the activation energy for this reaction?
A)
15kJ
done
clear
B)
30kJ
done
clear
C)
68 kJ
done
clear
D)
120 kJ
done
clear
View Answer play_arrow
question_answer 82) A 0.010 M solution of maleic acid, a monoprotic organic acid, is 14% ionised. What is \[{{K}_{a}}\] for maleic acid?
A)
\[2.3\times {{10}^{-3}}\]
done
clear
B)
\[2.3\times {{10}^{-4}}\]
done
clear
C)
\[2.0\times {{10}^{-4}}\]
done
clear
D)
\[2.0\times {{10}^{-6}}\]
done
clear
View Answer play_arrow
question_answer 83) What will happen to the volume of a bubble of air found under water in a lake, where the temperature is \[{{15}^{o}}C\] and the pressure is 1.5 atm, if the bubble then rises to the surface where the temperature is \[{{25}^{o}}C\]and the pressure is 1.0 atm?
A)
Its volume will become greater by a factor of 2.5
done
clear
B)
Its Volume will become greater by a factor of 1.6
done
clear
C)
Its volume will become greater by a factor of 1.1
done
clear
D)
Its volume will become smaller by a factor of 0.70
done
clear
View Answer play_arrow
question_answer 84) Which of these change (s) with time for a first order reaction? [A] Rate of reaction [B] Rate constant [C] Half-life
A)
[A] only
done
clear
B)
[C] only
done
clear
C)
[A] and [B] only
done
clear
D)
[B] and [C] only
done
clear
View Answer play_arrow
question_answer 85) What is the \[[{{H}^{+}}]\] in a 0.40 M solution of\[HOCl,\,{{K}_{a}}=3.5\times {{10}^{-8}}\]?
A)
\[1.4\times {{10}^{-8}}M\]
done
clear
B)
\[1.2\times {{10}^{-4}}M\]
done
clear
C)
\[1.9\times {{10}^{-4}}M\]
done
clear
D)
\[3.7\times {{10}^{-4}}M\]
done
clear
View Answer play_arrow
question_answer 86) Sodium chloride, NaCI, usually crystallizes in a face-centered cubic lattice. How many ions are in contact with any single \[N{{a}^{+}}\] ion?
A)
4
done
clear
B)
6
done
clear
C)
8
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 87) Which of these species has a standard enthalpy of formation equal to zero?
A)
\[{{F}_{2}}(g)\]
done
clear
B)
\[F(g)\]
done
clear
C)
\[HF(aq)\]
done
clear
D)
\[{{F}^{-}}(aq)\]
done
clear
View Answer play_arrow
question_answer 88) What is the osmotic pressure of a 0.0020 mol \[d{{m}^{-3}}\] sucrose \[({{C}_{12}}{{H}_{22}}{{O}_{11}})\] solution at \[{{20}^{o}}C\]? (Molar gas constant, \[R=8.314\,J{{K}^{-1}}mo{{l}^{-1}}\];\[1\,\,d{{m}^{3}}=0.001\,{{m}^{3}}\])
A)
4870 Pa
done
clear
B)
4.87 Pa
done
clear
C)
0.00487 Pa
done
clear
D)
0.33 Pa
done
clear
View Answer play_arrow
question_answer 89) Calculate the wavelength of light required to break the bond between two chlorine atoms in a chlorine molecule. The Cl-Cl bond energy is 243kJ\[mo{{l}^{-1}}\]. (\[h=6.6\times {{10}^{-34}}J.\,s;c=3\times {{10}^{8}}m/s;\]; Avogadros number \[=6.02\times {{10}^{23}}mo{{l}^{-1}}\]).
A)
\[8.18\times {{10}^{-31}}m\]
done
clear
B)
\[6.26\times {{10}^{-21}}m\]
done
clear
C)
\[4.93\times {{10}^{-7}}m\]
done
clear
D)
\[4.11\times {{10}^{-6}}m\]
done
clear
View Answer play_arrow
question_answer 90) As \[{{O}_{2}}(l)\] is cooled at 1 atm pressure, it freezes to form solid I at 54.5 K. At a lower temperature, solid I rearranges to solid II, which has a different crystal structure. Thermal measurements show that for the phase transition solid I to solid \[II,\,\Delta H=-743.1\,J\,mo{{l}^{-1}}\]and\[\Delta S=-17.0\,\,J{{K}^{-1}}\,mo{{l}^{-1}}\]. At what temperature are solids I and II in equilibrium?
A)
2.06 K
done
clear
B)
31.5 K
done
clear
C)
43.7 K
done
clear
D)
53.4 K
done
clear
View Answer play_arrow
question_answer 91) The hybridisation in \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\] is
A)
\[ds{{p}^{3}}\]
done
clear
B)
\[s{{p}^{3}}{{d}^{2}}\]
done
clear
C)
\[s{{p}^{3}}\]
done
clear
D)
\[{{d}^{2}}s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 92) Which of the following configuration of ions has zero CFSE in both strong and weal ligand fields?
A)
\[{{d}^{10}}\]
done
clear
B)
\[{{d}^{8}}\]
done
clear
C)
\[{{d}^{6}}\]
done
clear
D)
\[{{d}^{4}}\]
done
clear
View Answer play_arrow
question_answer 93) pi \[(\pi )\] bond is formed by the overlap of
A)
p-p orbitals
done
clear
B)
s-s orbitals
done
clear
C)
s-p orbitals
done
clear
D)
s-d orbitals
done
clear
View Answer play_arrow
question_answer 94) Which of the following is diamagnetic in nature?
A)
\[C{{o}^{3+}}\], octahedral complex with weak field ligands
done
clear
B)
\[C{{o}^{3+}}\], octahedral complex with strong field ligands
done
clear
C)
\[C{{o}^{2+}}\]in tetrahedral complex
done
clear
D)
\[C{{o}^{2+}}\]in square planar complex
done
clear
View Answer play_arrow
question_answer 95) Which of the following complexes has minimum magnitude of Ape?
A)
\[{{[Cr{{(CN)}_{6}}]}^{3-}}\]
done
clear
B)
\[{{[Co{{(NH)}_{3}})}_{6}}{{]}^{3+}}\]
done
clear
C)
\[{{[CoCl]}_{6}}{{]}^{3-}}\]
done
clear
D)
\[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 96) The polarity of the covalent bond among the following is maximum in
A)
F?F
done
clear
B)
0?F
done
clear
C)
N?F
done
clear
D)
C?F
done
clear
View Answer play_arrow
question_answer 97) Which one of the following ions will give a coloured solution?
A)
\[C{{u}^{+}}\]
done
clear
B)
\[F{{e}^{2+}}\]
done
clear
C)
\[Z{{n}^{2+}}\]
done
clear
D)
\[A{{g}^{+}}\]
done
clear
View Answer play_arrow
question_answer 98) On adding excess of \[N{{H}_{4}}OH\] to copper sulphate solution,
A)
a deep blue solution is obtained
done
clear
B)
a blue precipitate of \[Cu{{(OH)}_{2}}\] is obtained
done
clear
C)
a black precipitate of CuO is obtained
done
clear
D)
No change takes place
done
clear
View Answer play_arrow
question_answer 99) In \[Fe{{(CO)}_{5}}\], the \[Fe\leftarrow CO\,\sigma \] bond results by the overlap between filled sp hybrid orbital of C-atom of CO molecule and vacant hybrid orbital of Fe atom.
A)
\[{{d}^{2}}s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{3}}\]
done
clear
C)
\[ds{{p}^{3}}\]
done
clear
D)
\[ds{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 100) The bond angle formed by different hybrid orbitals are in the order
A)
\[s{{p}^{2}}>s{{p}^{3}}>sp\]
done
clear
B)
\[s{{p}^{3}}<s{{p}^{2}}>sp\]
done
clear
C)
\[s{{p}^{3}}>s{{p}^{2}}>sp\]
done
clear
D)
\[sp>s{{p}^{2}}>s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 101) The Wild Life (protection) Act, 1972 was first amended in
A)
1991
done
clear
B)
1995
done
clear
C)
2001
done
clear
D)
2007
done
clear
View Answer play_arrow
question_answer 102) In primary succession on rocks, the pioneer species are usually
A)
algae
done
clear
B)
fungi
done
clear
C)
lichens
done
clear
D)
bryophytes
done
clear
View Answer play_arrow
question_answer 103) The 10 per cent law is related to
A)
Mendelian genetics
done
clear
B)
non-Mendelian genetics
done
clear
C)
energy transfer from lower trophic level to higher trophic level
done
clear
D)
energy consumption during photosynthesis in \[{{C}_{4-}}\] plants
done
clear
View Answer play_arrow
question_answer 104) A nucleoside differs from a nucleotide in not having
A)
sugar
done
clear
B)
glucose
done
clear
C)
nitrogen base
done
clear
D)
phosphate group
done
clear
View Answer play_arrow
question_answer 105) Trichoderma species are potentially useful as
A)
biopesticides
done
clear
B)
biofertilizers
done
clear
C)
methanogens
done
clear
D)
vectors for genetic engineering
done
clear
View Answer play_arrow
question_answer 106) A pea plant parent having violet-coloured flowers with unknown genotype was crossed with a plant having white-coloured flowers. In the progeny, 50% of the flowers were violet and 50% were white. The genotypic constitution of the parent having violet-coloured flowers was
A)
homozygous
done
clear
B)
merozygous
done
clear
C)
heterozygous
done
clear
D)
hemizygous
done
clear
View Answer play_arrow
question_answer 107) If the total amount of adenine and thymine in a double-stranded DNA is 45%, the amount of guanine in this DNA will be
A)
22.5%
done
clear
B)
27.5%
done
clear
C)
45%
done
clear
D)
55%
done
clear
View Answer play_arrow
question_answer 108) Typhoid fever is caused by a species of
A)
Streptococcus
done
clear
B)
Staphylococcus
done
clear
C)
Salmonella
done
clear
D)
Mycobacterium
done
clear
View Answer play_arrow
question_answer 109) HIV is a member of a group of viruses called
A)
bacteriophages
done
clear
B)
geminiviruses
done
clear
C)
lysogenic viruses
done
clear
D)
retroviruses
done
clear
View Answer play_arrow
question_answer 110) The number of linkage group(s) present in Escherichia coli is
A)
one
done
clear
B)
two
done
clear
C)
four
done
clear
D)
seven
done
clear
View Answer play_arrow
question_answer 111) The exchange of segments of non-sister chromatids between chromosomes of a homologous pair is termed as
A)
transformation
done
clear
B)
translocation
done
clear
C)
crossing over
done
clear
D)
chromosomal aberration
done
clear
View Answer play_arrow
question_answer 112) Okazaki is known for his contribution to the understanding of
A)
transcription
done
clear
B)
translation
done
clear
C)
DNA replication
done
clear
D)
mutation
done
clear
View Answer play_arrow
question_answer 113) The beginning of understanding genetic transformation in bacteria was made by
A)
Frederick Griffith
done
clear
B)
Hershey and Chase
done
clear
C)
Watson and Crick
done
clear
D)
T.H. Morgan
done
clear
View Answer play_arrow
question_answer 114) The source of Taq polymeraise used in PCR is a
A)
thermophilic fungus
done
clear
B)
mesophilic fungus
done
clear
C)
thermophilic bacterium
done
clear
D)
halophilic bacterium
done
clear
View Answer play_arrow
question_answer 115) Which of the following is not used as bioweapon?
A)
Bacillus anthracis
done
clear
B)
Botulmum toxin
done
clear
C)
Bacillus thunngiensis toxin
done
clear
D)
Smallpox
done
clear
View Answer play_arrow
question_answer 116) Comparable to angiosparms, which of the following algae exhibits ddplontic life cycle?
A)
Spirogyra
done
clear
B)
Ectocarpus
done
clear
C)
Polysiphonia
done
clear
D)
Fucus
done
clear
View Answer play_arrow
question_answer 117) The cytoplasm of adjacent plant cells is connected to each other by
A)
plasmalemma
done
clear
B)
desmosome
done
clear
C)
plasmodesmata
done
clear
D)
plasmotubule
done
clear
View Answer play_arrow
question_answer 118) With the increase in diameter of the root, the effective RCF (Relative Centrifugal Force) at a fixed RPM (Revolutions Per Minute) will
A)
remain unaffected
done
clear
B)
increase
done
clear
C)
decrease
done
clear
D)
be lower at the bottom of the centrifugal tube
done
clear
View Answer play_arrow
question_answer 119) Which of the following amino acids has hydroxyl methyl group as its R group?
A)
Serine
done
clear
B)
Proline
done
clear
C)
Alanine
done
clear
D)
Arginine
done
clear
View Answer play_arrow
question_answer 120) Formation of both peptide and glycosidic bonds involves
A)
hydration
done
clear
B)
dehydration
done
clear
C)
esterification
done
clear
D)
acidlificaton
done
clear
View Answer play_arrow
question_answer 121) Which of the following events takes place during diplotene stage of prophase-I of meiosis?
A)
Compaction of chromosomes
done
clear
B)
Formation of synaptonemal complexes
done
clear
C)
Formation of recombinational nodules
done
clear
D)
Dissolution of synaptoi-emal complexes
done
clear
View Answer play_arrow
question_answer 122) The number of microtubules in a flagellum including those sharing three protofilaments with each other is
A)
11
done
clear
B)
20
done
clear
C)
22
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 123) Which of the following statement is correct?
A)
All bacteria are heterotrophic
done
clear
B)
Bacteria are either heterotrophic or chemoautotrophic
done
clear
C)
Bacteria can also be photoautotrophic
done
clear
D)
Bacteria are either photoautotrophic or chemoautotrophic
done
clear
View Answer play_arrow
question_answer 124) Apoplastic movement of water in plants occurs through
A)
casparian strips,
done
clear
B)
plasma membrane
done
clear
C)
intracellular spaces
done
clear
D)
plasmodesmata
done
clear
View Answer play_arrow
question_answer 125) Solute potential of a solution is always
A)
= 0
done
clear
B)
> 0
done
clear
C)
< 0
done
clear
D)
between 0-1
done
clear
View Answer play_arrow
question_answer 126) Simultaneous movement of two molecules across a membrane in the same direction is known as
A)
antiport
done
clear
B)
symport
done
clear
C)
uniport
done
clear
D)
biport
done
clear
View Answer play_arrow
question_answer 127) At the time of shedding, the number of nuclei present in an angiosperm, pollen grain is
A)
one
done
clear
B)
one or two
done
clear
C)
two or three
done
clear
D)
only two
done
clear
View Answer play_arrow
question_answer 128) Taxa differs from taxon due to this being
A)
a higher taxonomic category than taxon
done
clear
B)
lower taxonomic category than taxon
done
clear
C)
the plural of taxon
done
clear
D)
the singular of taxon
done
clear
View Answer play_arrow
question_answer 129) Which of the following wall layers of anther plays a predominant role in its dehiscence?
A)
Epidermis
done
clear
B)
Endothecium
done
clear
C)
Middle layers
done
clear
D)
Tapetum
done
clear
View Answer play_arrow
question_answer 130) The type of pollination involving transfer of pollen grains from anther to the stigma of the same flower is known as
A)
geitonogamy
done
clear
B)
xenogamy
done
clear
C)
autogamy
done
clear
D)
apogamy
done
clear
View Answer play_arrow
question_answer 131) The egg apparatus of angiosperm comprises
A)
an egg cell and two antipodals
done
clear
B)
an egg cell and two synergids
done
clear
C)
an egg cell and two polar nuclei
done
clear
D)
an egg cell and the central cell
done
clear
View Answer play_arrow
question_answer 132) The expression gynoecium is apocarpous implies that the
A)
gynoecium comprises only one pistil which is fused with the stamen
done
clear
B)
gynoecium comprises more than one carpel which are free from each other
done
clear
C)
gynoecium comprises only one carpel which is free
done
clear
D)
gynoecium comprises more than one carpel which are fused
done
clear
View Answer play_arrow
question_answer 133) A mixture containing DNA fragments, a,b,c and d, with molecular weights of a + b = c, a > b and d > c, was subjected to agarose gel electrophoresis. The positions of these fragments from cathode to anode sides of the gel would be.
A)
b,a,c,d
done
clear
B)
a,b,c,d
done
clear
C)
c,b,a,d
done
clear
D)
b,a,d,c
done
clear
View Answer play_arrow
question_answer 134) Which of the following DNA sequences qualifies to be designated as a palindrome?
A)
5 - GACCAG ? 3 in one strand
done
clear
B)
3 - GACCAG ? 5 in one strand
done
clear
C)
5 - GACGAG - 3 3 - CTGGTC ? 5
done
clear
D)
5 - AGCGCT ? 3 3 - TCGCGA ? 5
done
clear
View Answer play_arrow
question_answer 135) Restriction enzymes
A)
restrict elongation of DNA
done
clear
B)
cut DNA at specific locations
done
clear
C)
link together two pieces of DNA
done
clear
D)
restrict DNA replication
done
clear
View Answer play_arrow
question_answer 136) Which one of the following does not play any role in photosynthesis?
A)
Phycocyanin
done
clear
B)
Xanthophylls
done
clear
C)
Phycoerythrin
done
clear
D)
Anthocyanin
done
clear
View Answer play_arrow
question_answer 137) Biogas produced by fermentation of manure, sewage, cattle dung, etc predominantly comprises
A)
methane, nitrogen and hydrogen
done
clear
B)
methane and carbon dioxide
done
clear
C)
methane and carbon monoxide
done
clear
D)
methane and nitric oxide
done
clear
View Answer play_arrow
question_answer 138) Lactobacillus mediated conversion of milk to curd results because of
A)
coagulation and partial digestion of milk fats
done
clear
B)
coagulation and partial digestion of milk proteins
done
clear
C)
coagulation of milk proteins and complete digestion of milk fats
done
clear
D)
coagulation of milk fats and complete digestion of milk protein
done
clear
View Answer play_arrow
question_answer 139) The species of Saccharum originally grown in India was
A)
officinarum
done
clear
B)
barberi
done
clear
C)
bolardii
done
clear
D)
munja
done
clear
View Answer play_arrow
question_answer 140) Single cell protein refers to
A)
a specific protein extracted from pure culture of single type of cells
done
clear
B)
sources of mixed proteins extracted from pure or mixed culture of organisms or cells
done
clear
C)
proteins extracted from a single cell
done
clear
D)
a specific protein extracted from a single cell
done
clear
View Answer play_arrow
question_answer 141) The total number of species, that are known and described, range between
A)
0.5--1.0 million
done
clear
B)
1.1-1.2 million
done
clear
C)
1.7-1,8 million
done
clear
D)
2.5-3.0 million
done
clear
View Answer play_arrow
question_answer 142) Which of the following combinations is correct for wheat?
A)
Genus-Triticum, Family-Anacardiaceae, order-Poales, Class-Monocotyledonae
done
clear
B)
Genus-Tritfcum, Family-Poaceae, Order-Poales, Class-Dicotyledonae
done
clear
C)
Genus-Thtirum, Family-Poaceae, Order-Sapindales, Class-Monocotyledonae
done
clear
D)
Genus-7rriJcum, Family-Poaceae, Order-Poales, Class-Monocotyledonae
done
clear
View Answer play_arrow
question_answer 143) IUCN stands for
A)
Indian Union for Conservation of Nature
done
clear
B)
International Union for Conservation of Nature
done
clear
C)
Indian Union for Chemical Nomenclature
done
clear
D)
International Union for Conservation of Nutrients
done
clear
View Answer play_arrow
question_answer 144) A group of related genera, with still less number of similarities as compared to the genus and species, constitutes
A)
order
done
clear
B)
class
done
clear
C)
family
done
clear
D)
division
done
clear
View Answer play_arrow
question_answer 145) The timing of seasonal activities of plants in relation to change in environmental condition is termed as
A)
Dendrochronology
done
clear
B)
biological clock
done
clear
C)
lapse rate
done
clear
D)
Phenology
done
clear
View Answer play_arrow
question_answer 146) Who is considered as the father of Ecology in India?
A)
Ramdeo Misra
done
clear
B)
M.S. Swaminathan
done
clear
C)
P. Maheshwari
done
clear
D)
S.L. Mehta
done
clear
View Answer play_arrow
question_answer 147) A large regional unit characterized by a major vegetation type and associated fauna found in a specific climatic zone constitutes
A)
ecosystem
done
clear
B)
biological community
done
clear
C)
biome
done
clear
D)
habitat
done
clear
View Answer play_arrow
question_answer 148) Cold-blooded animals fall under the category of
A)
ectotherms
done
clear
B)
psychotherms
done
clear
C)
endotherms
done
clear
D)
thermophiles
done
clear
View Answer play_arrow
question_answer 149) figs belong to
A)
critical link species, as they form connecting link between trees and herbs
done
clear
B)
critical link species, as they establish essential link in the absorbance of nutrients from soil and organic residues .
done
clear
C)
keystone species, as they produce large quantity of fruits; and their protection leads to conservation of animals dependent on them
done
clear
D)
keystone species, as they have high degree of animal-dependent pollination
done
clear
View Answer play_arrow
question_answer 150) Which of the following is a mismatch with respect to inexhaustible natural resources?
A)
Solar energy
done
clear
B)
Water
done
clear
C)
Rainfall
done
clear
D)
Wind power
done
clear
View Answer play_arrow
question_answer 151) Mature erythrocytes cannot utilize glucose because they lack
A)
Golgi complex
done
clear
B)
enzymes
done
clear
C)
mitochondria
done
clear
D)
nucleus
done
clear
View Answer play_arrow
question_answer 152) Oral contraceptive pills help in birth control by
A)
killing sperms
done
clear
B)
killing ova
done
clear
C)
preventing ovulation
done
clear
D)
forming barrier between sperms and ova
done
clear
View Answer play_arrow
question_answer 153) Colour perception in man is due to
A)
rhodopsin pigment in rod cells
done
clear
B)
iodopsin pigment in cone cells
done
clear
C)
iodopsin pigment in rod cells
done
clear
D)
rhodopsin pigment in cone cells
done
clear
View Answer play_arrow
question_answer 154) Which of the following induces parturition?
A)
Vasopressin
done
clear
B)
Oxytocin
done
clear
C)
GH
done
clear
D)
TSH
done
clear
View Answer play_arrow
question_answer 155) Excess carbohydrates and proteins are stored in the body as
A)
amino acids
done
clear
B)
fats
done
clear
C)
starch
done
clear
D)
monosaccharides
done
clear
View Answer play_arrow
question_answer 156) Which of the following vitamins has some physiological effects similar to those of parathormone?
A)
Vitamin-A
done
clear
B)
Vitamin-D
done
clear
C)
Vitamin-C
done
clear
D)
Vitamin-B
done
clear
View Answer play_arrow
question_answer 157) Which of the following microbes is used for commercial production of ethanol?
A)
Clostridium botulinum
done
clear
B)
Streptococcus
done
clear
C)
Trichoderma polysporum
done
clear
D)
Saccharomyces cerevisiae
done
clear
View Answer play_arrow
question_answer 158) Appropriate measures to reduce overall green house gas emissions are the commitments of the
A)
Montreal Protocol
done
clear
B)
Environment Act
done
clear
C)
Kyoto Protocol
done
clear
D)
Earth Summit
done
clear
View Answer play_arrow
question_answer 159) Which one of the following pairs of diseases is viral as well as transmitted by mosquitoes?
A)
Elephantiasis and dengue
done
clear
B)
Yellow fever and sleeping sickness
done
clear
C)
Encephalitis and sleeping sickness
done
clear
D)
Yellow fever and dengue
done
clear
View Answer play_arrow
question_answer 160) Classification of organisms based on evolutionary as well as genetic relationships is called
A)
biosystematics
done
clear
B)
phonetics
done
clear
C)
numerical taxonomy
done
clear
D)
cladistics
done
clear
View Answer play_arrow
question_answer 161) Hyaline cartilage does not have
A)
fibres
done
clear
B)
lacunae
done
clear
C)
cells
done
clear
D)
blood capillaries
done
clear
View Answer play_arrow
question_answer 162) All enzymes are proteins. This statement is now modified because an apparent exception to this biological truth is
A)
arylsulphatase
done
clear
B)
dehydrogenase
done
clear
C)
ribozyme
done
clear
D)
nitroreductase
done
clear
View Answer play_arrow
question_answer 163) Ageing of the skin results in
A)
an increase in collagen and elastic fibres a
done
clear
B)
decrease in activity of sebaceous glands
done
clear
C)
a thickening of the skin
done
clear
D)
an increase in toenail growth
done
clear
View Answer play_arrow
question_answer 164) The example of pivot joint is
A)
hip joint
done
clear
B)
metacarpophalangeal joint
done
clear
C)
ankle joint
done
clear
D)
radio-ulnar joint
done
clear
View Answer play_arrow
question_answer 165) MS- The major function of the in tervertebral discs is to
A)
absorb shock
done
clear
B)
string the vertebrae together
done
clear
C)
prevent injuries
done
clear
D)
prevent hyperextension
done
clear
View Answer play_arrow
question_answer 166) Bile contribution to digestion is
A)
nucleic acid metabolism
done
clear
B)
phagocytosis
done
clear
C)
emulsification of dietary lipids
done
clear
D)
carbohydrate digestion
done
clear
View Answer play_arrow
question_answer 167) The first fossil evidence of life dates from about
A)
4.0 billion years ago
done
clear
B)
3.6 billion years ago
done
clear
C)
4.5 billion years ago
done
clear
D)
2.5 billion years ago
done
clear
View Answer play_arrow
question_answer 168) What will be the correct gene expression pathway?
A)
Gene-mRNA-Transcription-Translation- Protein
done
clear
B)
Transcription-Gene-Translation-mRNA- Protein
done
clear
C)
Gene-Transcription-mRNA-Translation- Protein
done
clear
D)
Gene-Translation-mRNA-Transcription- Protein
done
clear
View Answer play_arrow
question_answer 169) Cloning is a process, where
A)
gene is cloned in an animal
done
clear
B)
fragments of DNA are transferred from one organism to another, usually carried on a DNA vector
done
clear
C)
fragments of DNA cloned in the same organism using carrier
done
clear
D)
DNA is cloned in plants
done
clear
View Answer play_arrow
question_answer 170) Enzyme that cleaves nucleic acids within the polynucleotide chain is known as
A)
endonuclease
done
clear
B)
exonuclease
done
clear
C)
arylsulphatase
done
clear
D)
phosphotriesterase
done
clear
View Answer play_arrow
question_answer 171) Phylum?Mollusca can be distinguished from other invertebrates by the presence of
A)
bilateral symmetry and exoskeleton
done
clear
B)
a mantle and gills
done
clear
C)
shell and non-segmented body
done
clear
D)
a mantle and non-segmented body
done
clear
View Answer play_arrow
question_answer 172) A logistic growth curve depicting a population that is limited by a definite carrying capacity is shaped like the letter
A)
J
done
clear
B)
L
done
clear
C)
M
done
clear
D)
S
done
clear
View Answer play_arrow
question_answer 173) The urge to inhale in human results from
A)
rising \[{{P}_{C{{O}_{2}}}}\]
done
clear
B)
rising \[{{P}_{{{O}_{2}}}}\]
done
clear
C)
falling \[{{P}_{C{{O}_{2}}}}\]
done
clear
D)
falling \[{{P}_{{{O}_{2}}}}\]
done
clear
View Answer play_arrow
question_answer 174) Rotenone is a
A)
bioherbicide
done
clear
B)
commonly used biofertilizer
done
clear
C)
bioinsecticide
done
clear
D)
juvenile hormone
done
clear
View Answer play_arrow
question_answer 175) A phenomenon when parasite parasitizes themselves is known as
A)
hyperparasitism
done
clear
B)
parasitoids
done
clear
C)
monoxenous parasitism
done
clear
D)
polyxenous parasitism
done
clear
View Answer play_arrow
question_answer 176) Phosphorus-32 emits
A)
\[\alpha \]-particles
done
clear
B)
\[\beta \]-particles
done
clear
C)
\[\gamma \]-particles
done
clear
D)
X-rays
done
clear
View Answer play_arrow
question_answer 177) The long and short arms of chromosome are designated respectively as
A)
p and q arms
done
clear
B)
q and p arms
done
clear
C)
m and p arms
done
clear
D)
\[l\] and s arms
done
clear
View Answer play_arrow
question_answer 178) The amino acid that acts as a carrier of ammonia from skeletal muscle to liver is
A)
alanine
done
clear
B)
methionine
done
clear
C)
arginine
done
clear
D)
glutamine
done
clear
View Answer play_arrow
question_answer 179) The major cause of evolution of genes and protein is
A)
point mutation
done
clear
B)
chromosomal aberration
done
clear
C)
sexual reproduction
done
clear
D)
gene duplication and divergence
done
clear
View Answer play_arrow
question_answer 180) The type of ecosystem with the highest mean plant productivity is
A)
desert
done
clear
B)
temperate grassland
done
clear
C)
tropical rain forest
done
clear
D)
tundra
done
clear
View Answer play_arrow
question_answer 181) A common means of sympatric speciation is
A)
polyploidy
done
clear
B)
temporal segregation of breeding season
done
clear
C)
spatial segregation of mating sites
done
clear
D)
imposition of geographic barrier
done
clear
View Answer play_arrow
question_answer 182) Bacillus thuringiensis is used to control
A)
bacterial pathogens
done
clear
B)
fungal pathogens
done
clear
C)
nematodes
done
clear
D)
insect pests
done
clear
View Answer play_arrow
question_answer 183) Bell-shaped polygonal pyramid indicates
A)
high percentage of young individuals
done
clear
B)
moderate percentage of young individuals
done
clear
C)
low percentage of young individuals
done
clear
D)
low percentage of old individuals
done
clear
View Answer play_arrow
question_answer 184) Probiotics are
A)
cancer inducing microbes
done
clear
B)
safe antibiotics
done
clear
C)
food allergens
done
clear
D)
five microbial food supplements
done
clear
View Answer play_arrow
question_answer 185) Molecules that bear charged groups of opposite polarity are known as
A)
Zwitter ions
done
clear
B)
cations
done
clear
C)
anions
done
clear
D)
negative ions
done
clear
View Answer play_arrow
question_answer 186) Hotspots of biodiversity means
A)
areas of the earth that contain many endemic species
done
clear
B)
species severes as proxy for entire communities in particular area
done
clear
C)
species in particular niche/area
done
clear
D)
species diversity at particular area
done
clear
View Answer play_arrow
question_answer 187) Polio is caused by a
A)
bacteriophage
done
clear
B)
virus with a single-stranded RNA
done
clear
C)
virus with a single-stranded DNA
done
clear
D)
virus with double-stranded DNA
done
clear
View Answer play_arrow
question_answer 188) Disadvantage of MRI is its inability to image
A)
bone
done
clear
B)
parts of brain
done
clear
C)
spinal cord
done
clear
D)
cancerous tissues
done
clear
View Answer play_arrow
question_answer 189) Which of the following is an r-strategist?
A)
Human
done
clear
B)
Insect
done
clear
C)
Rhinoceros
done
clear
D)
Whale
done
clear
View Answer play_arrow
question_answer 190) The internal cavity commonly formed by cell division prior to gastrulation is the
A)
enteron
done
clear
B)
blastopore
done
clear
C)
blastocoel
done
clear
D)
coelom
done
clear
View Answer play_arrow
question_answer 191) Mendels principle of segregation means that the germ cells always receive
A)
one pair of alleles
done
clear
B)
one quarter of the genes
done
clear
C)
one of the paired alleles
done
clear
D)
any pairs of alleles
done
clear
View Answer play_arrow
question_answer 192) Aggregates of lymphoid tissue present in the distal portion of the small intestine are known
A)
villi
done
clear
B)
Peyers patches
done
clear
C)
rugae
done
clear
D)
choroid plexus
done
clear
View Answer play_arrow
question_answer 193) Microfilaments in eukaryotic cells are made up of
A)
actin
done
clear
B)
albumin
done
clear
C)
globulin
done
clear
D)
fibrin
done
clear
View Answer play_arrow
question_answer 194) The membranous areas between the cranial bones of the fetal skull are called
A)
areolas
done
clear
B)
foramina
done
clear
C)
sutures
done
clear
D)
fontanelle
done
clear
View Answer play_arrow
question_answer 195) The predominant antibody in saliva is
A)
IgG
done
clear
B)
IgA
done
clear
C)
IgM
done
clear
D)
IgD
done
clear
View Answer play_arrow
question_answer 196) In order for the blood to flow from right ventricle to left ventricle in mammalian heart, it must flow through
A)
right ventricle, pulmonary arteries, lungs, pulmonary veins, left atrium
done
clear
B)
right ventricle, pulmonary veins, lungs, pulmonary arteries, left atrium
done
clear
C)
right ventricle, right atrium, lungs, pulmonary veins, left atrium
done
clear
D)
right ventricle, systemic aorta, lungs, pulmonary veins, left atrium,
done
clear
View Answer play_arrow
question_answer 197) Air bladder is present in
A)
Chondrichthyes
done
clear
B)
star fishes
done
clear
C)
Actinopterygii
done
clear
D)
flying fishes
done
clear
View Answer play_arrow
question_answer 198) Which of the following is the key intermediate compound linking glycolysis to the Krebs cvcle?
A)
NADH
done
clear
B)
ATP
done
clear
C)
Acetyl Co-A
done
clear
D)
Malic acid
done
clear
View Answer play_arrow
question_answer 199) Urea synthesis takes place primarily in liver because
A)
\[N{{H}_{3}}\] and \[C{{O}_{2}}\] are present in liver only
done
clear
B)
hormone ADH is found in liver only
done
clear
C)
enzyme arginase is present in liver only
done
clear
D)
kidney is smaller than liver
done
clear
View Answer play_arrow
question_answer 200) A particular enzyme molecule interacts with the specific substrate molecule is explained by
A)
enzyme-substrate concept
done
clear
B)
activation energy concept
done
clear
C)
destroyed and re-synthesised concept
done
clear
D)
lock and key concept
done
clear
View Answer play_arrow