question_answer 1) Which of the following principles is being used in Sonar Technology?
A)
Reflection of ultrasonic waves
done
clear
B)
Newtons laws of motion
done
clear
C)
Reflection of electromagnetic waves
done
clear
D)
Laws of thermodynamics
done
clear
View Answer play_arrow
question_answer 2) What is the dimension of surface tension?
A)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{\text{1}}}{{\text{T}}^{\text{o}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
B)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{\text{1}}}{{\text{T}}^{\text{-1}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
C)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{o}}{{\text{T}}^{\text{-2}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
D)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{1}}{{\text{L}}^{o}}{{\text{T}}^{\text{-2}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
View Answer play_arrow
question_answer 3)
The speed-time graph of a particle moving along a solid curve is shown below. The distance traversed by the panicle from t =0 tor =3 is
A)
\[\frac{10}{2}s\]
done
clear
B)
\[\frac{10}{4}s\]
done
clear
C)
\[\frac{10}{3}s\]
done
clear
D)
\[\frac{10}{5}s\]
done
clear
E)
None of these
done
clear
View Answer play_arrow
question_answer 4) Which of the following is correct relation between an arbitrary vector A and null vector O?
A)
\[\text{A+O+A }\!\!\times\!\!\text{ O=A}\]
done
clear
B)
\[\text{A+O+A }\!\!\times\!\!\text{ O}\ne \text{A}\]
done
clear
C)
\[\text{A+O+A }\!\!\times\!\!\text{ O=O}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 5) An object is being thrown at a speed of 20 m/s in a direction 45° above the horizontal- The rime taken by the object to return to the same level is
A)
20/g
done
clear
B)
20 g
done
clear
C)
20\[\sqrt{2}\]/g
done
clear
D)
20\[\sqrt{2}\]g
done
clear
View Answer play_arrow
question_answer 6) An object is moving on a plane surface with uniform velocity 10ms1 in presence of a force 10 N. The frictional force between the object and the surface is
A)
IN
done
clear
B)
-ION
done
clear
C)
10 N
done
clear
D)
100 N
done
clear
View Answer play_arrow
question_answer 7)
A body of mass M starts sliding down on the inclined plane where the critical angle is Z ACB =30° as shown in figure. The coefficient of kinetic friction will be
A)
\[Mg/\sqrt{3}\]
done
clear
B)
\[\sqrt{3}Mg\]
done
clear
C)
\[\sqrt{3}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 8) In non-inertial frame, the second law of motion is written as
A)
\[F=ma\]
done
clear
B)
\[F=ma+{{F}_{P}}\]
done
clear
C)
\[F=ma-{{F}_{P}}\]
done
clear
D)
\[F=2ma\] Where \[{{F}_{p}}\] is pseudo-force while a is the acceleration of the body relative to non-inertial frame.
done
clear
View Answer play_arrow
question_answer 9) The work done by an applied variable force\[F=x+{{x}^{3}}\] from\[x=o\,m\,to\,x\,=2,\,m\] where \[x\] is displacement, is
A)
6J
done
clear
B)
8J
done
clear
C)
10 J
done
clear
D)
12 J
done
clear
View Answer play_arrow
question_answer 10) The coefficient of restitute, e, for a perfectly elastic collision is
A)
0
done
clear
B)
-1
done
clear
C)
1
done
clear
D)
\[\infty \]
done
clear
View Answer play_arrow
question_answer 11) A particle of mass mi moves with velocity\[{{v}_{1}}\] and collides with another particle at rest of equal mass. The velocity of the second panicle after the elastic collision is
A)
\[2{{v}_{1}}\]
done
clear
B)
\[{{v}_{1}}\]
done
clear
C)
\[-{{v}_{1}}\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 12) The centre of mass of a solid cone along the line from the centre of the base to the vertex is at
A)
one-fourth of the height
done
clear
B)
one-third of the height
done
clear
C)
one-fifth of the height
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 13) A solid cylinder is rolling without slipping on a plane having inclination \[\theta \] and The coefficient of static friction\[{{\mu }_{s.}}\] The relation between \[\theta \] and \[{{\mu }_{s.}}\] is
A)
tan\[\theta >3{{\mu }_{s}}\]
done
clear
B)
tan \[\theta \le 3{{\mu }_{s}}\]
done
clear
C)
tan\[\theta <3{{\mu }_{s}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 14) The reduced mass of two panicles having masses m and 2m is
A)
2m
done
clear
B)
3m
done
clear
C)
2m/3
done
clear
D)
m/2
done
clear
View Answer play_arrow
question_answer 15) Which of the following graphs shows the variation of acceleration due to gravity g with depth h from the surface of the earth?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 16) At what altitude (\[h\]) above the earths surface would the acceleration due to gravity be one fourth of its value at the earths surface?
A)
h=R
done
clear
B)
h=4R
done
clear
C)
h=2R
done
clear
D)
n=16R where, R is the radius of the earth.
done
clear
View Answer play_arrow
question_answer 17) According to CE van der Waal, the in teratomic potential veries with the average in teratomic distance (R) as
A)
\[{{R}^{-1}}\]
done
clear
B)
\[{{R}^{-2}}\]
done
clear
C)
\[{{R}^{-4}}\]
done
clear
D)
\[{{R}^{-6}}\]
done
clear
View Answer play_arrow
question_answer 18) A sphere of radius 3 cm is subjected to a pressure of 100 an n. Its volume decreases by 0.3 cc. What will be its bulk modulus?
A)
\[4\pi \times {{10}^{5}}\] atm
done
clear
B)
\[4\pi \times {{103}^{4}}\] atm
done
clear
C)
\[4\pi \times {{10}^{6}}\]atm
done
clear
D)
\[4\pi \times {{10}^{8}}\]
done
clear
E)
None of these
done
clear
View Answer play_arrow
question_answer 19) A vertical tank with depth H is full with water. A hole is made on one side of the walls at a depth h below the water surface. At what distance from the foot of the wall does the emerging stream of water strike the foot?
A)
\[\sqrt{h(H-h)}\]
done
clear
B)
\[2\sqrt{h/(H-h)}\]
done
clear
C)
\[2(H-h)\sqrt{h/(H-h)}\]
done
clear
D)
\[\sqrt{2h/(H-h)}\]
done
clear
View Answer play_arrow
question_answer 20) The mean free path of collision of gas molecules varies with its diameter of the molecules as
A)
\[d{{-}^{1}}\]
done
clear
B)
\[d{{-}^{2}}\]
done
clear
C)
\[d{{-}^{3}}\]
done
clear
D)
\[d{{-}^{4}}\]
done
clear
View Answer play_arrow
question_answer 21)
Consider two insulated chambers (A, B) of same volume connected by a closed knob, S. 1 mole of perfect gas is confined in chamber What is the change in entropy of gas when knob S is opened? R = 8.31 J mo1 -1K-1
A)
1.46 J/K
done
clear
B)
3.46 J/K
done
clear
C)
5.46 J/K
done
clear
D)
7.46 J/K
done
clear
View Answer play_arrow
question_answer 22) A Carnot engine has efficiency 25%. It operates between reservoirs of constant temperatures with temperature difference of 80°C. What is the temperature of the low-temperature reservoir?
A)
\[-25{}^\circ C\]
done
clear
B)
\[25{}^\circ C\]
done
clear
C)
\[-33{}^\circ C\]
done
clear
D)
\[33{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 23) During the phenomenon of resonance
A)
the amplitude of oscillation becomes large
done
clear
B)
the frequency of oscillation becomes large
done
clear
C)
the time period of oscillation becomes large
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 24) The longitudinal wave can be observed in
A)
elastic media
done
clear
B)
inelastic media
done
clear
C)
Both of the above
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 25) The two waves of the same frequency moving in the same direction give rise to
A)
beats
done
clear
B)
interference
done
clear
C)
stationary waves
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 26) Domestic electrical wiring has three wires
A)
positive, negative and neutral
done
clear
B)
positive, negative and earth
done
clear
C)
live, neutral and earth
done
clear
D)
positive, negative and live
done
clear
View Answer play_arrow
question_answer 27) Which of the following is not true?
A)
For a point charge, the electrostatic potential varies as 1/r
done
clear
B)
For a dipole, the potential depends on the position vector and dipole moment vector
done
clear
C)
The electric dipole potential varies as 1/r at large distance
done
clear
D)
For a point charge, the electrostatic field varies as 1/r2
done
clear
View Answer play_arrow
question_answer 28) The mobility of charge carriers increases with
A)
increase in the average collision time
done
clear
B)
increase in the electric field
done
clear
C)
increase in the mass of the charge carriers
done
clear
D)
decrease in the charge of the mobile carriers
done
clear
View Answer play_arrow
question_answer 29) When an AC voltage is applied to a L-C-R circuit, which of the following is true?
A)
\[I\]and V are out of phase with each other In R
done
clear
B)
\[I\] and V are in phase in L with in C, they are out of phase
done
clear
C)
\[I\] and V are out of phase in both, C and L
done
clear
D)
\[I\]and V are out of phase in L and in phase In C
done
clear
View Answer play_arrow
question_answer 30) For a medium with permittivity\[\varepsilon \]and permeability\[\mu ,\]the velocity of light is given by
A)
\[\sqrt{\mu /\varepsilon }\]
done
clear
B)
\[\sqrt{\mu \varepsilon }\]
done
clear
C)
\[1/\sqrt{\varepsilon /\mu }\]
done
clear
D)
\[\sqrt{\varepsilon /\mu }\]
done
clear
View Answer play_arrow
question_answer 31) In optical fibres, the refractive index of the core is
A)
greater than that of the cladding
done
clear
B)
equal to that of the cladding
done
clear
C)
smaller than that of the cladding
done
clear
D)
in dependent of that of the cladding
done
clear
View Answer play_arrow
question_answer 32) For a wavelength of light\[\lambda \]and scattering object of size a, all wavelengths are scattered nearly equally, if
A)
\[a=\lambda \]
done
clear
B)
\[a>>\lambda \]
done
clear
C)
\[a<<\lambda \]
done
clear
D)
\[a+\ge \lambda \]
done
clear
View Answer play_arrow
question_answer 33) For a telescope having \[{{f}_{o}}\]as the focal length of the objective and \[{{f}_{e}}\] as the focal length of the eyepiece, the length of the telescope tube is
A)
\[{{f}_{e}}\]
done
clear
B)
\[{{f}_{o}}-{{f}_{e}}\]
done
clear
C)
\[{{f}_{o}}\]
done
clear
D)
\[{{f}_{o}}+{{f}_{e}}\]
done
clear
View Answer play_arrow
question_answer 34) If two sources have a randomly varying phase difference \[\phi (t),\] the resultant intensity will be given by
A)
\[1/2{{I}_{o}}\]
done
clear
B)
\[{{I}_{o}}/2\]
done
clear
C)
\[2{{I}_{o}}\]
done
clear
D)
\[{{I}_{o}}/\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 35) For an aperture of size a illuminated by a parallel beam of light having wavelength \[\lambda ,\] the Fresnel distance is
A)
\[\approx a/\lambda \]
done
clear
B)
\[\approx {{a}^{2}}/\lambda \]
done
clear
C)
\[\approx {{a}^{2}}\lambda \]
done
clear
D)
\[\approx a/{{\lambda }^{2}}\]
done
clear
View Answer play_arrow
question_answer 36) The maximum kinetic energy of the photoelectrons varies
A)
inversely with the intensity and is independent of the frequency of the incident radiation
done
clear
B)
inversely with the frequency and is independent of the intensity of the incident radiation
done
clear
C)
linearly with the frequency and the intensity of the incident radiation
done
clear
D)
linearly with the frequency and is independent of the intensity of the incident radiation
done
clear
View Answer play_arrow
question_answer 37) The work function for A1, K and Pt is 4.28eV, 2.30eV and 5.65eV respectively. Their respective threshold frequencies would be
A)
\[\text{PtA1K}\]
done
clear
B)
\[\text{A1PtK}\]
done
clear
C)
\[\text{KA1Pt}\]
done
clear
D)
\[\text{A1KPt}\]
done
clear
View Answer play_arrow
question_answer 38) When helium nuclei bombard beryllium nuclei, then
A)
electrons are emitted
done
clear
B)
protons are emitted
done
clear
C)
neutrons are emitted
done
clear
D)
protons and neutrons are emitted
done
clear
View Answer play_arrow
question_answer 39) When two nuclei (with A = 8) join to form a heavier nucleus, the binding energy (BE) per nucleon of the heavier nuclei is
A)
more than the BE per nucleon of the lighter nuclei
done
clear
B)
same as the BE per nucleon of the lighter nuclei
done
clear
C)
less than the BE per nucleon of the lighter nuclei
done
clear
D)
double the BE per nucleon of the lighter nuclei
done
clear
View Answer play_arrow
question_answer 40) In a revrese-biased p-n junction, when the applied bias voltage is equal to the breakdown voltage, then
A)
current remains constant while voltage increases sharply
done
clear
B)
voltage remains constant while current increases sharply
done
clear
C)
current and voltage increase
done
clear
D)
current and voltage decrease
done
clear
View Answer play_arrow
question_answer 41) A charged cloud system produces an electric field in the air near the earths surface. A particle of charge \[-2\times {{10}^{-9}}C\] is acted on by a downward electrostatic force of \[3\times {{10}^{-6}}N\] when placed in this field. The gravitational and electrostatic force, respectively, exerted on a proton placed in this field are
A)
\[\text{1}\text{.64 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-26}}}\,\text{N,2}\text{.4 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-16}\,}}\text{N}\]
done
clear
B)
\[\text{1}\text{.64 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-26}}}\,\text{N,1}\text{.5 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-3}\,}}\text{N}\]
done
clear
C)
\[\text{1}\text{.56 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-18}}}\,\text{N,2}\text{.4 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-16}}}\text{N}\]
done
clear
D)
\[\text{1}\text{.5 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-3}}}\,\text{N,2}\text{.4 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-16}}}\text{N}\]
done
clear
View Answer play_arrow
question_answer 42) The frequency of oscillation of an electric dipole moment having dipole moment p and rotational inertia\[I,\] oscillating in a uniform electric field E is given by
A)
\[(1/2\pi )\sqrt{I/PE}\]
done
clear
B)
\[(1/2\pi )\sqrt{{{P}^{E}}/I}\]
done
clear
C)
\[(2\pi )\sqrt{{{P}^{E}}/I}\]
done
clear
D)
\[(2\pi )\sqrt{/I{{P}^{E}}}\]
done
clear
View Answer play_arrow
question_answer 43) What is the net charge on a conducting sphere of radius 10 cm? Given that the electric field 15 cm from the centre of the sphere is equal to \[3\times {{10}^{3}}\]N/C and is directed inward
A)
\[-7.5\times {{10}^{-5}}C\]
done
clear
B)
\[-7.5\times {{10}^{-9}}C\]
done
clear
C)
\[-7.5\times {{10}^{-5}}C\]
done
clear
D)
\[-7.5\times {{10}^{-9}}C\]
done
clear
View Answer play_arrow
question_answer 44) How many\[1\mu F\] capacitors must be connected in parallel to store a charge of 1 C with a potential of 110 V across the capacitors?
A)
990
done
clear
B)
900
done
clear
C)
9090
done
clear
D)
909
done
clear
View Answer play_arrow
question_answer 45) A 1250 W heater operates at 115 V. What is the resistance of the heating coil?
A)
1.6\[\Omega \]
done
clear
B)
13.5\[\Omega \]
done
clear
C)
1250\[\Omega \]
done
clear
D)
10.6\[\Omega \]
done
clear
View Answer play_arrow
question_answer 46) A proton travelling at \[23{}^\circ \]w.r.t. the direction of a magnetic field of strength 2.6 mT experiences a magnetic force of \[6.5\times {{10}^{-17}}\,N\]what is the speed of the proton?
A)
\[2\times {{10}^{-5}}\,m/s\]
done
clear
B)
\[4\times {{10}^{-5}}\,m/s\]
done
clear
C)
\[6\times {{10}^{-5}}\,m/s\]
done
clear
D)
\[6\times {{10}^{-5}}\,m/s\]
done
clear
View Answer play_arrow
question_answer 47) What uniform magnetic field applied perpendicular to a beam of electrons moving at 1.3x106 m/s, is required to make the electrons travel in a circular arc of radius 0.35 m?
A)
\[2.1\times {{10}^{-5}}\,G\]
done
clear
B)
\[6\times {{10}^{-5}}\,T\]
done
clear
C)
\[2.1\times {{10}^{-5}}\,T\]
done
clear
D)
\[6\times {{10}^{-5}}\,G\]
done
clear
View Answer play_arrow
question_answer 48) A transformer has 500 primary turns and 10 secondary turns. If the secondary has a resistive load of 15 \[\Omega \] the currents in the primary and secondary respectively, are
A)
\[\text{0}\text{.16A,3}\text{.2 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-3}}}\text{A}\]
done
clear
B)
\[\text{3}\text{.2 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-3}}}\text{A,}\,\text{0}\text{.16A}\]
done
clear
C)
\[\text{0}\text{.16A, }\!\!\times\!\!\text{ }\,\text{0}\text{.16A}\]
done
clear
D)
\[\text{3}\text{.2 }\!\!\times\!\!\text{ 1}\,\text{0}{{\text{.}}^{\text{-3}}}\text{A,}\,\text{3}\text{.2 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-3}}}\text{A}\]
done
clear
View Answer play_arrow
question_answer 49) For a radio signal to travel 150 km from the transmitter to a receiving antenna, it takes
A)
\[\text{5 }\!\!\times\!\!\text{ 1}\,\text{0}{{\text{.}}^{\text{-4}}}s\]
done
clear
B)
\[\text{4}\text{.5 }\!\!\times\!\!\text{ 1}\,{{\text{0}}^{\text{-3}}}s\]
done
clear
C)
\[\text{5 }\!\!\times\!\!\text{ 1}\,{{\text{0}}^{\text{-8}}}s\]
done
clear
D)
\[\text{4}\text{.5 }\!\!\times\!\!\text{ 1}\,{{\text{0}}^{\text{-6}}}s\]
done
clear
View Answer play_arrow
question_answer 50) In Youngs double-slit experiment, if the distance between the slits is halved and the distance between the slits and the screen is doubled, the fringe width becomes
A)
half
done
clear
B)
double
done
clear
C)
four times
done
clear
D)
eight times
done
clear
View Answer play_arrow
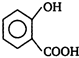
question_answer 51) In the given structure of a compound, the correct various bond moments direction involving are shown as
A)
\[Br\underline{\leftarrow }N\underline{\leftarrow }C{{H}_{2}}\underline{\to }Si{{H}_{2}}\underline{\leftarrow }C{{H}_{2}}\underline{\to }O\underline{\leftarrow }C{{H}_{3}}\]
done
clear
B)
\[Br\underline{\leftarrow }N\underline{\leftarrow }C{{H}_{2}}\underline{\leftarrow }Si{{H}_{2}}\underline{\leftarrow }C{{H}_{2}}\underline{\to }O\underline{\leftarrow }C{{H}_{3}}\]
done
clear
C)
\[Br\underline{\leftarrow }N\underline{\to }C{{H}_{2}}\underline{\leftarrow }Si{{H}_{2}}\to C{{H}_{2}}\underline{\to }O\underline{\leftarrow }C{{H}_{3}}\]
done
clear
D)
\[Br\underline{\leftarrow }N\underline{\to }C{{H}_{2}}\underline{\to }Si{{H}_{2}}\underline{\leftarrow }C{{H}_{2}}\underline{\to }O\underline{\to }C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 52)
For the given alkane
A)
1,1 dimethyl-5-ethyl octane
done
clear
B)
6-ethyl-2-methyl nonane
done
clear
C)
4-ethyl-8-methyl nonane
done
clear
D)
2-methyl, -6-propyl octane
done
clear
View Answer play_arrow
question_answer 53) Which will undergo fastest 82 substitution reaction when treated with NaOH?
A)
\[{{H}_{5}}{{C}_{2}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\mathop{C}}\,}}\,-Br\]
done
clear
B)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,}}\,-Br\]
done
clear
C)
\[H-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ {{C}_{2}}{{H}_{5}} \end{smallmatrix}}{\mathop{C}}\,}}\,-Br\]
done
clear
D)
\[H-\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{C}}\,}}\,-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 54)
A)
hexane
done
clear
B)
cyclohexane
done
clear
C)
cyclohexylcyclohexane
done
clear
D)
cyclohexylether
done
clear
View Answer play_arrow
question_answer 55) Most stable carbocation is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 56) Which one of the following alkylbromides undergoes most rapid solvolysis in methanol solution to give corresponding methyl ether?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 57)
In the conversion of
A)
\[{{H}_{2}}/Pt\]
done
clear
B)
Zn-Hg/HCl
done
clear
C)
\[Li/N{{H}_{3}}\]
done
clear
D)
\[NaB{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 58) Which is not the correct statement about RNA and DNA?
A)
DNA is active in virus where RNA never appears in virus
done
clear
B)
DNA exists as dimer while RNA is usually single stranded
done
clear
C)
DNA contains deoxyribose as its sugar and RNA contains ribose
done
clear
D)
RNA contains uracil in place of thymine (found in DNA) as a base
done
clear
View Answer play_arrow
question_answer 59) What is the nature of glucose-glucose linkage in starch that makes its so susceptible to acid hydrolysis?
A)
Starch is hemiacetal
done
clear
B)
Starch is acetal
done
clear
C)
Starch is polymer
done
clear
D)
Starch contains only few molecules of glucose
done
clear
View Answer play_arrow
question_answer 60)
In the conversion
A)
(i) \[SOC{{l}_{2}}\] (ii) \[N_{3}^{-}\] (iii) H20, heat
done
clear
B)
(i) \[SOC{{l}_{2}}\] (ii) \[N{{H}_{3}}\]
done
clear
C)
(i) \[SOC{{l}_{2}}\] (ii)\[N{{H}_{3}}\] (iii) Heat
done
clear
D)
(i) \[SOC{{l}_{2}}\] (ii) KCN (iii) \[LiAlH\]
done
clear
View Answer play_arrow
question_answer 61) In the reaction \[2{{H}_{2}}{{O}_{2}}\xrightarrow{{}}2{{H}_{2}}O+{{O}_{2}}\]
A)
oxygen is oxidised only
done
clear
B)
oxygen is reduced only
done
clear
C)
oxygen is neither oxidised nor reduced
done
clear
D)
oxygen is both oxidised and reduced
done
clear
View Answer play_arrow
question_answer 62) Which one of the following is not acid-base conjugate pair?
A)
\[HONO,\,NO_{2}^{-}\]
done
clear
B)
\[C{{H}_{3}}NH_{3}^{+},C{{H}_{3}}N{{H}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}COOH,{{C}_{6}}{{H}_{5}}CO{{O}^{-}}\]
done
clear
D)
\[{{H}_{3}}{{O}^{+}},O{{H}^{-}}\]
done
clear
View Answer play_arrow
question_answer 63) Which one of the following has the strongest O - O bond?
A)
\[O_{2}^{+}\]
done
clear
B)
\[O_{2}^{0}\]
done
clear
C)
\[O_{2}^{-}\]
done
clear
D)
\[O_{2}^{2-}\]
done
clear
View Answer play_arrow
question_answer 64) For the reactions \[{{I}_{2}}(aq){{I}_{2}}\] (oil); equilibrium constant is \[{{K}_{1}}\] \[{{I}_{2}}\] (oil) \[{{I}_{2}}\] (ether); equilibrium constant is \[{{K}_{2}}\] for the reaction \[{{I}_{2}}(aq){{I}_{2}}\] (ether); equilibrium constant is \[{{K}_{3}}\] The relation between \[{{K}_{1}},{{K}_{2}},{{K}_{3}}\] is
A)
\[{{K}_{3}}={{K}_{1}}+{{K}_{2}}\]
done
clear
B)
\[{{K}_{3}}={{K}_{1}}{{K}_{2}}\]
done
clear
C)
\[{{K}_{3}}={{K}_{1}}/{{K}_{2}}\]
done
clear
D)
\[{{K}_{3}}={{K}_{2}}/{{K}_{1}}\]
done
clear
View Answer play_arrow
question_answer 65) The geometry of electron pairs around I in \[I{{F}_{5}}\] is
A)
octahedral
done
clear
B)
trigonal bipyramidal
done
clear
C)
square pyramidal
done
clear
D)
pentagonal planar
done
clear
View Answer play_arrow
question_answer 66) Which statement is/are not correct?
A)
Rate of an exothermic reaction increases with temperature
done
clear
B)
Solubility of NaOH increases with temperature
done
clear
C)
\[{{K}_{p}}\]for \[{{N}_{2}}(g)+3{{H}_{2}}(g)2N{{H}_{3}}(g)\]increases with increase in pressure
done
clear
D)
For gaseous reaction \[2\,B\xrightarrow{{}}A\,;{{K}_{p}}\] is smaller than \[{{K}_{e}}\]
done
clear
View Answer play_arrow
question_answer 67) Which of the following changes require an oxidising agent?
A)
\[2{{S}_{2}}O_{3}^{2-}{{S}_{4}}O_{6}^{2-}\]
done
clear
B)
\[Z{{n}^{2+}}Zn\]
done
clear
C)
\[Cl{{O}^{-}}C{{l}^{-}}\]
done
clear
D)
\[S{{O}_{3}}SO_{4}^{2-}\]
done
clear
View Answer play_arrow
question_answer 68) Given the following reactions involving A, B, C and D (i) \[C+{{B}^{+}}\to {{C}^{+}}+B\] (ii) \[{{A}^{+}}+D\to \] No reaction (iii) \[{{C}^{+}}+A\to \] No reaction (iv) \[D+{{B}^{+}}\to {{D}^{+}}+B\] The correct arrangement of A, B, C, D in order of their decreasing ability as reducing agent
A)
D > B > C > A
done
clear
B)
A > C > D > B
done
clear
C)
C > A > B > D
done
clear
D)
C > A > D > B
done
clear
View Answer play_arrow
question_answer 69) Which ion has the largest radius?
A)
\[S{{e}^{2-}}\]
done
clear
B)
\[{{F}^{-}}\]
done
clear
C)
\[{{O}^{2-}}\]
done
clear
D)
\[R{{b}^{+}}\]
done
clear
View Answer play_arrow
question_answer 70) Which is correct statement about \[C{{r}_{2}}O_{7}^{2-}\]structure?
A)
It has neither Cr - Cr bonds nor O - O bonds
done
clear
B)
It has one Cr - Cr bond and six O - O bonds
done
clear
C)
It has no Cr - Cr bond and has six O - O bonds
done
clear
D)
It has one Cr - Cr bond and seven Cr - O bonds
done
clear
View Answer play_arrow
question_answer 71) Which reaction, with the following values of \[\Delta H,\,\,\Delta S\]at 400 K is spontaneous and endothermic?
A)
\[\Delta H=-48\,kJ;\,\,\Delta S=+135\,J/K\]
done
clear
B)
\[\Delta H=-48\,kJ;\,\,\Delta S=-135\,J/K\]
done
clear
C)
\[\Delta H=+48\,kJ;\,\,\Delta S=+135\,J/K\]
done
clear
D)
\[\Delta H=+48\,kJ;\,\,\Delta S=-135\,J/K\]
done
clear
View Answer play_arrow
question_answer 72) The correct decreasing order of dipole moment in \[C{{H}_{3}}Cl,\,C{{H}_{3}}\] Br and \[C{{H}_{3}}F\] is
A)
\[C{{H}_{3}}F>C{{H}_{3}}Cl>C{{H}_{3}}Br\]
done
clear
B)
\[C{{H}_{3}}F>C{{H}_{3}}Br>C{{H}_{3}}Cl\]
done
clear
C)
\[C{{H}_{3}}Cl>C{{H}_{3}}F>C{{H}_{3}}Br\]
done
clear
D)
\[C{{H}_{3}}Cl>C{{H}_{3}}Br>C{{H}_{3}}F\]
done
clear
View Answer play_arrow
question_answer 73) Given exothermic reaction \[CoCl_{4}^{2-}(aq)+6{{H}_{2}}O(l){{[Co{{({{H}_{2}}O)}_{6}}]}^{2+}}\]\[+4C{{l}^{-}}\] Which one of the following will decrease the equilibrium concentration of \[CoCl_{4}^{2-}\]?
A)
Addition of \[HCl\]
done
clear
B)
Addition of \[Co{{(N{{O}_{3}})}_{2}}\]
done
clear
C)
The solution is diluted with water
done
clear
D)
The temperature is increased
done
clear
View Answer play_arrow
question_answer 74) Hydrogen is prepared from \[{{H}_{2}}O\] by adding
A)
Ca, which acts as reducing agent
done
clear
B)
Al, which acts as oxidising agent
done
clear
C)
Ag, which acts as reducing agent
done
clear
D)
Au, which acts as oxidising agent
done
clear
View Answer play_arrow
question_answer 75) For preparing a buffer solution of pH = 7.0, which buffer system will you choose?
A)
\[{{H}_{3}}P{{O}_{4}},{{H}_{2}}PO_{4}^{-}\]
done
clear
B)
\[{{H}_{2}}PO_{4}^{-},PO_{4}^{3-}\]
done
clear
C)
\[HPO_{4}^{2-},PO_{4}^{3-}\]
done
clear
D)
\[{{H}_{3}}P{{O}_{4}},\,PO_{4}^{3-}\]
done
clear
View Answer play_arrow
question_answer 76) Which element undergoes disproportionation in water?
A)
\[C{{l}_{2}}\]
done
clear
B)
\[{{F}_{2}}\]
done
clear
C)
K
done
clear
D)
Cs
done
clear
View Answer play_arrow
question_answer 77) Which one of the following species acts only as a base?
A)
\[{{H}_{2}}S\]
done
clear
B)
\[H{{S}^{-}}\]
done
clear
C)
\[{{S}^{2-}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 78) For the following reaction \[{{C}_{6}}{{H}_{12}}{{O}_{6}}(aq)+{{H}_{2}}(g){{C}_{6}}{{H}_{14}}{{O}_{6}}(aq)\] Which one of the following is not affected by the addition of catalyst?
A)
Rate of forward reaction
done
clear
B)
Rate of backward reaction
done
clear
C)
Time required to reach the equilibrium
done
clear
D)
Spontaneity
done
clear
View Answer play_arrow
question_answer 79) Which is not the correct statement?
A)
The \[{{S}_{8}}\] ring is not planar
done
clear
B)
Oxygen is more electronegative than sulphur
done
clear
C)
\[S{{F}_{4}}\] exists, but \[O{{F}_{4}}\] does not exist
done
clear
D)
\[S{{O}_{3}}\] and \[SO_{3}^{2-}\] both have trigonal planar geometry
done
clear
View Answer play_arrow
question_answer 80) Which can exist both as diastereoisomer and enantiomer?
A)
\[{{[Pt{{(en)}_{3}}]}^{4+}}\]
done
clear
B)
\[{{[Pt{{(en)}_{2}}ClBr]}^{2+}}\]
done
clear
C)
\[{{[Ru{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]}^{0}}\]
done
clear
D)
\[{{[PtC{{l}_{2}}B{{r}_{2}}]}^{0}}\]
done
clear
View Answer play_arrow
question_answer 81) Number of isomeric forms (constitutional and stereoisomers) for \[[Rh{{(en)}_{2}}(N{{O}_{2}})\,{{(SCN)}^{+}}\] are
A)
three
done
clear
B)
six
done
clear
C)
nine
done
clear
D)
twelve
done
clear
View Answer play_arrow
question_answer 82) For transition metal octahedral complexes, the choice between high spin and low spin electronic configurations arises only for
A)
\[{{d}^{1}}\] to \[{{d}^{3}}\] complexes
done
clear
B)
\[{{d}^{4}}\] to \[{{d}^{7}}\]complexes
done
clear
C)
\[{{d}^{7}}\]to \[{{d}^{9}}\] complexes
done
clear
D)
\[{{d}^{1}},\,{{d}^{2}}\]and \[{{d}^{8}}\] complexes
done
clear
View Answer play_arrow
question_answer 83) For a chemical reaction of the type \[AB,\,K=2.0\] and \[BC,\,K=0.01\]Equilibrium constant for the reaction \[2C2A\] is
A)
25
done
clear
B)
50
done
clear
C)
2500
done
clear
D)
\[4\times {{10}^{-4}}\]
done
clear
View Answer play_arrow
question_answer 84)
A chemical reaction proceeds into the following steps Step I, \[2AX\] fast Step II, \[X+BY\] slow Step III, \[Y+B\]Product fast
The rate law for the overall reaction is
A)
Rate \[=k{{[A]}^{2}}\]
done
clear
B)
Rate \[=k{{[B]}^{2}}\]
done
clear
C)
Rate \[=k[A][B]\]
done
clear
D)
Rate \[=k{{[A]}^{2}}[B]\]
done
clear
View Answer play_arrow
question_answer 85) A solution is 0.1 M with respect to \[A{{g}^{+}},C{{a}^{2+}},M{{g}^{2+}}\]and \[A{{l}^{3+}}\], which will precipitate at lowest concentration of \[[PO_{4}^{3-}]\] when solution of \[N{{a}_{3}}P{{O}_{4}}\] is added?
A)
\[A{{g}_{3}}P{{O}_{3}}({{K}_{sp}}=1\times {{10}^{-6}})\]
done
clear
B)
\[C{{a}_{3}}{{(P{{O}_{4}})}_{2}}({{K}_{sp}}=1\times {{10}^{-33}})\]
done
clear
C)
\[M{{g}_{3}}{{(P{{O}_{4}})}_{2}}({{K}_{sp}}=1\times {{10}^{-24}})\]
done
clear
D)
\[AlP{{O}_{4}}({{K}_{sp}}=1\times {{10}^{-20}})\]
done
clear
View Answer play_arrow
question_answer 86) In Tollens test, aldehydes
A)
are oxidised to acids
done
clear
B)
are reduced to alcohol
done
clear
C)
neither reduced nor oxidised
done
clear
D)
precipitate Ag+ as AgCl
done
clear
View Answer play_arrow
question_answer 87) The half-life time of 2g sample of radioactive nuclide X is 15 min. The half-life time of 1g sample of X is
A)
7.5 min
done
clear
B)
15 min
done
clear
C)
22.5 min
done
clear
D)
30 min
done
clear
View Answer play_arrow
question_answer 88) Given a gas phase reaction \[2A(g)+B(g)C(g)+D(g)\]Which one of the following changes will
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 89) affect the value of \[{{K}_{c}}\]?
A)
Addition of inert gas
done
clear
B)
Addition of catalyst
done
clear
C)
Addition of reactants
done
clear
D)
Increasing in temperature
done
clear
View Answer play_arrow
question_answer 90) Lowest \[p{{K}_{a}}\] is associated with Monobromination of 2-methylbutane gives how many distinct isomers?
A)
One
done
clear
B)
Two
done
clear
C)
Three
done
clear
D)
Four
done
clear
View Answer play_arrow
question_answer 91) \[\alpha -(D)\]glucose\[\beta -(D)\]glucose, equilibrium constant for this is 1.8. The percentage of \[\alpha \]- glucose at equilibrium is
A)
35.7
done
clear
B)
55.6
done
clear
C)
44.4
done
clear
D)
64.3
done
clear
View Answer play_arrow
question_answer 92) Equal weights of \[C{{H}_{4}}\] and \[{{H}_{2}}\] are mixed in a container at \[{{25}^{o}}C\]. Fraction of total pressure exerted by methane is
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{1}{3}\]
done
clear
C)
\[\frac{1}{9}\]
done
clear
D)
\[\frac{8}{9}\]
done
clear
View Answer play_arrow
question_answer 93) In which one of the given formulae of xenon compounds there are five \[\alpha \]-bonds and three \[\pi \]-bonds in it?
A)
XeFO
done
clear
B)
\[Xe{{F}_{2}}{{O}_{2}}\]
done
clear
C)
\[Xe{{F}_{3}}{{O}_{2}}\]
done
clear
D)
\[Xe{{F}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 94) More acidic than ethanol is
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}C{{O}_{2}}C{{H}_{2}}C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{2}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 95) Of the following compounds, the oxime of which shows geometrical isomerism, is
A)
acetone
done
clear
B)
diethylketone
done
clear
C)
formaldehyde
done
clear
D)
benzaldehyde
done
clear
View Answer play_arrow
question_answer 96) Decreasing order of reactivity of hydrogen halide acids in the conversion of \[ROH\to RX\]is
A)
\[HCl>HBr>HI>HF\]
done
clear
B)
\[HI>HBr>HCl>HF\]
done
clear
C)
\[HF>HCl>HBr>HI\]
done
clear
D)
\[HF>HBr>HI>HCl\]
done
clear
View Answer play_arrow
question_answer 97) Which is correct statement?
A)
o-nitrobenzoic acid is stronger than 3, 5 dinitrobenzoic acid in \[{{H}_{2}}O\]
done
clear
B)
Branched carboxylic acids are more acidic than unbranched acids
done
clear
C)
is stronger acid than
done
clear
D)
Butanoic acid is stronger acid than succinic acid
done
clear
View Answer play_arrow
question_answer 98) Maximum efficiency of a commercial refrigerator which operates between -10°C (inside temperature) and \[{{25}^{o}}C\](outside temperature) is
A)
13.3%
done
clear
B)
11.45%
done
clear
C)
24.75%
done
clear
D)
20%
done
clear
View Answer play_arrow
question_answer 99) \[1\times {{10}^{-3}}m\] solution of \[Pt{{(N{{H}_{3}})}_{4}}C{{l}_{4}}\] in \[{{H}_{2}}O\]shows depression in freezing point by\[{{0.0054}^{o}}C\]. The structure of the compound will be [Given, \[{{K}_{f}}({{H}_{2}}O)=1.860\,\,k{{m}^{-1}}\]]
A)
\[[Pt{{(N{{H}_{3}})}_{4}}]C{{l}_{4}}\]
done
clear
B)
\[[Pt{{(N{{H}_{3}})}_{3}}]C{{l}_{3}}\]
done
clear
C)
\[[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]C{{l}_{2}}\]
done
clear
D)
\[[Pt(N{{H}_{3}})C{{l}_{3}}]Cl\]
done
clear
View Answer play_arrow
question_answer 100) The typical range of molar enthalpies for the strongest intermolecular (hydrogen) bonds is
A)
200 - 300 kJ
done
clear
B)
300 - 500 kJ
done
clear
C)
4 - 25 kJ
done
clear
D)
4 - 25 J
done
clear
View Answer play_arrow
question_answer 101) The most important factor which determined the increase in human population in India during the 20th century.
A)
Natality
done
clear
B)
Mortality
done
clear
C)
Immigration
done
clear
D)
Emigration
done
clear
View Answer play_arrow
question_answer 102) Vascular bundles in monocotyledons are considered closed because
A)
xylem is surrounded all around by phloem
done
clear
B)
there are no vessels with perforations
done
clear
C)
a bundle sheath surrounds each bundle
done
clear
D)
there is no secondary growth
done
clear
View Answer play_arrow
question_answer 103) When there are two haploid nuclei per cell in some fungi before the formation of diploid, this stage is called
A)
diplotene
done
clear
B)
diplophase
done
clear
C)
dikaryophase
done
clear
D)
dikaryote
done
clear
View Answer play_arrow
question_answer 104) In blood group typing in human, if an allele contributed by one parent is \[{{I}^{4}}\] and an allele contributed by the other parent is \[i\], the resulting blood group of the offspring will be
A)
A
done
clear
B)
B
done
clear
C)
AB
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 105) A population growing in a habitat with limited resources shows four phases of growth in the following sequence.
A)
Acceleration - Deceleration - Lag phase - Asymptote
done
clear
B)
Asymptote - Acceleration - Deceleration Lag phase
done
clear
C)
Lag phase - Acceleration - Deceleration - Asymptote
done
clear
D)
Acceleration - Lag phase - Deceleration -Asymptote
done
clear
View Answer play_arrow
question_answer 106) Necrosis in crops is due to the deficiency of
A)
Ca, K, S and Mo
done
clear
B)
N, K, S and Mo
done
clear
C)
N.S. Fe and Zn
done
clear
D)
Mg, S, Mn and Ca
done
clear
View Answer play_arrow
question_answer 107) Presence of bundle sheath is a characteristic of:
A)
xerophytic plants
done
clear
B)
members of grass family
done
clear
C)
\[{{C}_{4}}\]-plants
done
clear
D)
\[{{C}_{3}}\]-plants
done
clear
View Answer play_arrow
question_answer 108) Which one of the following would not lead to formation of clones?
A)
Double fertilization
done
clear
B)
Apomixis
done
clear
C)
Vegetative reproduction
done
clear
D)
Tissue culture
done
clear
View Answer play_arrow
question_answer 109) A plant species which has been exploited for the production of Hirudin is
A)
Brassica napus
done
clear
B)
Zea mays
done
clear
C)
Solanun nigrum
done
clear
D)
Oryza sativa
done
clear
View Answer play_arrow
question_answer 110) The variation/difference in the off springs of a species from their parents constitutes an important component of
A)
genetics
done
clear
B)
speciation
done
clear
C)
species fixation
done
clear
D)
heredity
done
clear
View Answer play_arrow
question_answer 111) If two pea plants having red (dominant) coloured flowers with unknown genotypes are crossed, 75% of the flowers are red and 25% are white. The genotypic constitution of the parents having red coloured flowers will
A)
be both homozygous
done
clear
B)
one homozygous and other heterozygous
done
clear
C)
both heterozygous
done
clear
D)
both hemizygous
done
clear
View Answer play_arrow
question_answer 112) If the total amount of adenine and thymine in a double-stranded DNA is 60%, the amount of guanine in this DNA will be
A)
15%
done
clear
B)
20%
done
clear
C)
30%
done
clear
D)
40%
done
clear
View Answer play_arrow
question_answer 113) The protein products of the following Bt toxin genes cry I Ac and cry II Ab are responsible for controlling
A)
bollworm
done
clear
B)
roundworm
done
clear
C)
moth
done
clear
D)
fruit fly
done
clear
View Answer play_arrow
question_answer 114) In a flowering plant, the pollen tube first arrives in
A)
egg
done
clear
B)
an antipodal cell
done
clear
C)
a synergid
done
clear
D)
central cell
done
clear
View Answer play_arrow
question_answer 115) A peculiar odour that prevails in marshy areas and cow-sheds is on account of a gas produced by
A)
mycoplasma
done
clear
B)
archaebacteria
done
clear
C)
slime moulds
done
clear
D)
cyanobacteria
done
clear
View Answer play_arrow
question_answer 116) A germplasm collection is a
A)
collection of specimens of all the species of an area in a herbarium or botanical garden
done
clear
B)
collection of modern varieties of a crop
done
clear
C)
collection of plants or seeds having diverse alleles of all genes in a crop
done
clear
D)
collection of seeds or pollen of rare and threatened species of a group or area
done
clear
View Answer play_arrow
question_answer 117) Walter Sutton is famous for his contribution to
A)
genetic engineering
done
clear
B)
to tipotency
done
clear
C)
quantitative genetics
done
clear
D)
chromosomal theory of inheritance
done
clear
View Answer play_arrow
question_answer 118) The reaction, Amino acid + ATP\[\to \] Aminoacyl AMP + P-P depicts
A)
amino acid assimilation
done
clear
B)
amino acid transformation
done
clear
C)
amino acid activation
done
clear
D)
amino acid translocation
done
clear
View Answer play_arrow
question_answer 119) The problem of blindness in poor countries can be taken care of by using the following
A)
golden rice
done
clear
B)
transgenic tomato
done
clear
C)
transgenic maize
done
clear
D)
Bt brinjal
done
clear
View Answer play_arrow
question_answer 120) The transcription of any gene is the indication of its
A)
induction
done
clear
B)
activity
done
clear
C)
stimulation
done
clear
D)
hypersensitivity
done
clear
View Answer play_arrow
question_answer 121) In C4-plants, the bundle sheath cells
A)
have thin walls to facilitate gaseous exchange
done
clear
B)
have large intercellular spaces
done
clear
C)
are rich in PE.P carboxylase
done
clear
D)
have a high density of chloroplasts
done
clear
View Answer play_arrow
question_answer 122) In root nodules of legumes, leghaemoglobin is important because it
A)
transports oxygen to the root nodule
done
clear
B)
acts as an oxygen scavenger
done
clear
C)
provides energy to the nitrogen fixing bacterium
done
clear
D)
acts as a catalyst in transamination
done
clear
View Answer play_arrow
question_answer 123) Darwin judged the fitness of an individual by
A)
ability to defend itself
done
clear
B)
strategy to obtain food
done
clear
C)
number of offsprings
done
clear
D)
dominance over other individuals
done
clear
View Answer play_arrow
question_answer 124) Which of the following statements is wrong?
A)
Pollen grains remain viable for several months because their outer covering is made of sporopollenin
done
clear
B)
No enzyme can degrade sporopollenin
done
clear
C)
Pollen grains are well represented in fossil strata due to sporopollenin
done
clear
D)
Pollen wall has cavities containing proteins
done
clear
View Answer play_arrow
question_answer 125) In plant biotechnology, PEG is used in
A)
protoplast isolation
done
clear
B)
cell culture preparation
done
clear
C)
protoplast fusion
done
clear
D)
hardening
done
clear
View Answer play_arrow
question_answer 126) A regulatory body working under Mo EF for the release of transgenic crops is
A)
NBPGR
done
clear
B)
GEAC
done
clear
C)
NSC
done
clear
D)
NIPGR
done
clear
View Answer play_arrow
question_answer 127) Analogous structures are
A)
anatomically different but performing similar functions
done
clear
B)
anatomically similar but performing different functions
done
clear
C)
anatomically similar and functioning similarly
done
clear
D)
anatomically different and functioning differently
done
clear
View Answer play_arrow
question_answer 128) A polygenic trait is controlled by 3 genes A, B and C. In a cross AaBbCc \[\times \]AaBbCc, the phenotypic ratio of the off springs was observed as 1 : 6 : x : 20 : x : 6 : 1 What is the possible value of x?
A)
3
done
clear
B)
9
done
clear
C)
15
done
clear
D)
25
done
clear
View Answer play_arrow
question_answer 129) A transgenic rice (golden rice) has been developed for increased content of
A)
vitamin-A
done
clear
B)
vitamin-B;
done
clear
C)
vitamin-C
done
clear
D)
vitamin-D
done
clear
View Answer play_arrow
question_answer 130) When the conditions are dry, a grass leaf curls inward to minimize water loss due to presence of
A)
thick cuticle
done
clear
B)
large xylem cavities
done
clear
C)
parallel venation
done
clear
D)
bulliform cells
done
clear
View Answer play_arrow
question_answer 131) Long, ribbon-like pollen grains are seen in some
A)
aquatic plants
done
clear
B)
wind-pollinated grasses
done
clear
C)
gymnosperms
done
clear
D)
bird-pollinated flowers
done
clear
View Answer play_arrow
question_answer 132) At present, the concentration of \[C{{O}_{2}}\] in the atmosphere is about
A)
100 ppm
done
clear
B)
240 ppm
done
clear
C)
380 ppm
done
clear
D)
520 ppm
done
clear
View Answer play_arrow
question_answer 133) Littoral zone is located along the
A)
high mountains
done
clear
B)
sea
done
clear
C)
rivers
done
clear
D)
desert
done
clear
View Answer play_arrow
question_answer 134) Which of the following is used as a bioweapon?
A)
Bacillus subtilis
done
clear
B)
Bacillus licheniformis
done
clear
C)
Bacillus thuringiensis
done
clear
D)
Bacillus anthracis
done
clear
View Answer play_arrow
question_answer 135) The chromosome constitution 2n - 2 of an organism represents
A)
monosomic
done
clear
B)
nullisomic
done
clear
C)
haploid
done
clear
D)
trisomic
done
clear
View Answer play_arrow
question_answer 136) Meristem culture is practised in horticulture to get
A)
somaclonal variation
done
clear
B)
haploids
done
clear
C)
virus-free plants
done
clear
D)
slow-growing callus
done
clear
View Answer play_arrow
question_answer 137) Tendrils in plants are an example of
A)
convergent evolution
done
clear
B)
radiation
done
clear
C)
divergent evolution
done
clear
D)
co-evolution
done
clear
View Answer play_arrow
question_answer 138) Haemoglobin is
A)
an oxygen carrier in human blood
done
clear
B)
a protein used as food supplement
done
clear
C)
an oxygen scavenger in root nodules
done
clear
D)
a plant protein with high lysine content
done
clear
View Answer play_arrow
question_answer 139) Stomatal opening is affected by
A)
nitrogen concentration, carbon dioxide concentration and light
done
clear
B)
carbon dioxide concentration, temperature and light
done
clear
C)
nitrogen concentration, light an temperature
done
clear
D)
carbon dioxide concentration, nitrogen concentration and temperature
done
clear
View Answer play_arrow
question_answer 140) Taxonomic hierarchy refers to
A)
step-wise arrangement of all categories for classification of plants and animals
done
clear
B)
a group of senior taxonomists, who decide the nomenclature of plants and animals
done
clear
C)
a list of botanists or zoologists, who have worked on taxonomy of a species or group
done
clear
D)
classification of a species based on fossil record
done
clear
View Answer play_arrow
question_answer 141) Which of the following get accumulated in the vacuoles of guard cells during stomatal opening?
A)
Water, calcium and magnesium
done
clear
B)
Starch, potassium and chloride ions
done
clear
C)
Malate, sodium and potassium ions
done
clear
D)
Malate, potassium and chloride ions
done
clear
View Answer play_arrow
question_answer 142) Which of the following is the most accepted theory for movement of water through plants?
A)
Cohesion theory
done
clear
B)
Capillarity
done
clear
C)
Passive transport
done
clear
D)
Root pressure
done
clear
View Answer play_arrow
question_answer 143) Scutellum in a caryopsis represents
A)
outermost layer of endosperm
done
clear
B)
a sheath that protects the radicle
done
clear
C)
the place where the seed is attached to raphe
done
clear
D)
a cotyledon
done
clear
View Answer play_arrow
question_answer 144) In an annual ring, the light coloured part is known as
A)
early wood
done
clear
B)
late wood
done
clear
C)
heartwood
done
clear
D)
sapwood
done
clear
View Answer play_arrow
question_answer 145) Natural cytokinins are synthesized in tissue that are
A)
senescent
done
clear
B)
dividing rapidly
done
clear
C)
storing food material
done
clear
D)
differentiating
done
clear
View Answer play_arrow
question_answer 146) Resemblance of one organism to another for protection and hiding is
A)
mimicry
done
clear
B)
predation
done
clear
C)
adaptation
done
clear
D)
camouflage
done
clear
View Answer play_arrow
question_answer 147) Spirochaetes is are
A)
a class of insects
done
clear
B)
a class of viruses
done
clear
C)
bacteria
done
clear
D)
fungi
done
clear
View Answer play_arrow
question_answer 148) The metachromatic granules are
A)
present in plant cells at metaphase stage
done
clear
B)
inclusion bodies in bacteria
done
clear
C)
produced in insects during metamorphosis
done
clear
D)
chromatophores in animals skin
done
clear
View Answer play_arrow
question_answer 149) The rough endoplasmic reticulum (RER) in the cells are because of the presence of
A)
mitochondria associated with ER
done
clear
B)
ribosomes on the surface of ER
done
clear
C)
volutin granules on the surface of ER
done
clear
D)
sulphur granules on the surface of ER
done
clear
View Answer play_arrow
question_answer 150) Elaioplasts store
A)
starch
done
clear
B)
proteins
done
clear
C)
fats
done
clear
D)
essential amino acids
done
clear
View Answer play_arrow
question_answer 151) Aggregates of lymphoid tissue present in the distal portion of the small intestine are known as
A)
villi
done
clear
B)
Peyers patches
done
clear
C)
rugae
done
clear
D)
choroid plexus
done
clear
View Answer play_arrow
question_answer 152) Mendels principle of segregation means that the germ cells always receive
A)
one pair of alleles
done
clear
B)
one quarter of the genes
done
clear
C)
one of the paired alleles
done
clear
D)
any pair of alleles
done
clear
View Answer play_arrow
question_answer 153) Rotenone is a
A)
bioherbicide
done
clear
B)
commonly used biofertilizer
done
clear
C)
bioinsecticide
done
clear
D)
juvenile hormone
done
clear
View Answer play_arrow
question_answer 154) Which of the following vitamins has some physiological effects similar to those of parathormone?
A)
Vitamin-A
done
clear
B)
Vitamin-D
done
clear
C)
Vitamin-C
done
clear
D)
Vitamin-B
done
clear
View Answer play_arrow
question_answer 155) Somatostatin
A)
stimulates glucagon release while inhibits insulin release
done
clear
B)
stimulates release of insulin and glucagon
done
clear
C)
inhibits release of insulin and glucagon
done
clear
D)
inhibits glucagon release while stimulates insulin release
done
clear
View Answer play_arrow
question_answer 156) Hiccups can be best described as
A)
forceful sudden expiration
done
clear
B)
jerky incomplete inspiration
done
clear
C)
vibration of the soft palate during breathing
done
clear
D)
sign of indigestion
done
clear
View Answer play_arrow
question_answer 157) ELISA assay
A)
uses complement mediated cell lysis
done
clear
B)
uses a radiolabelled second antibody
done
clear
C)
involves addition of substrate which is convened into coloured end product
done
clear
D)
requires red blood cells
done
clear
View Answer play_arrow
question_answer 158) Complete competitors cannot coexist is true for
A)
character displacement
done
clear
B)
competitive exclusion
done
clear
C)
primary succession
done
clear
D)
secondary sucession
done
clear
View Answer play_arrow
question_answer 159) mRNA directs the building of proteins through a sequence of
A)
introns
done
clear
B)
codons
done
clear
C)
exons
done
clear
D)
anticodons
done
clear
View Answer play_arrow
question_answer 160) Foramen ovale
A)
connects the two atria in the foetal hear
done
clear
B)
is a condition in which the heart valves do not completely close
done
clear
C)
is a shallow depression in the interventricular septum
done
clear
D)
is a connection between the pulmonary trunk and the aorta in the foetus
done
clear
View Answer play_arrow
question_answer 161) Which of the following is a Gram negative bacterium?
A)
Escherichia coli
done
clear
B)
Bacillus subtilis
done
clear
C)
Streptomyces coelicolor
done
clear
D)
Ampycolatopsis orientalis
done
clear
View Answer play_arrow
question_answer 162) What is meant by the term Darwin fitness?
A)
The ability to survive and reproduce
done
clear
B)
High aggressiveness
done
clear
C)
Healthy appearance
done
clear
D)
Physical strength
done
clear
View Answer play_arrow
question_answer 163) Absence of one sex chromosome causes
A)
Turners syndrome
done
clear
B)
Klinefelters syndrome
done
clear
C)
Downs syndrome
done
clear
D)
Tay-Sachs syndrome
done
clear
View Answer play_arrow
question_answer 164) Comparing small and large cells, which statement is correct?
A)
Small cells have a small surface area per volume ratio
done
clear
B)
Exchange rate of nutrients is fast with large cells
done
clear
C)
Small cells have a large surface area per volume ratio
done
clear
D)
Exchange rate of nutrients is slow with small cells
done
clear
View Answer play_arrow
question_answer 165) Which one of the following animals shows discontinuous distribution?
A)
Green muscles
done
clear
B)
Bats
done
clear
C)
Lung fishes
done
clear
D)
Pacific salmons
done
clear
View Answer play_arrow
question_answer 166) The number of autosomes in human primary spermatocyte is
A)
46
done
clear
B)
44
done
clear
C)
23
done
clear
D)
22
done
clear
View Answer play_arrow
question_answer 167) The most abundant molecule in cell is
A)
water
done
clear
B)
carbohydrate
done
clear
C)
lipid
done
clear
D)
protein
done
clear
View Answer play_arrow
question_answer 168) How many chromosomes will the cell have at\[{{G}_{1}}\], after S and after M-phase respectively, if it has 14 chromosomes at interphase?
A)
14, 14, 7
done
clear
B)
14, 14, 14
done
clear
C)
7, 7, 7
done
clear
D)
7, 14, 14
done
clear
View Answer play_arrow
question_answer 169) The Golgi apparatus
A)
is found only in animals
done
clear
B)
is found in prokaryotes
done
clear
C)
is a site of rapid ATP production
done
clear
D)
modifies and packages proteins
done
clear
View Answer play_arrow
question_answer 170) Glycolysis
A)
takes place in the mitochondria
done
clear
B)
produces no ATP
done
clear
C)
has no connection with electron transport chain
done
clear
D)
reduces two molecules of \[NA{{D}^{+}}\] for every glucose molecule processed
done
clear
View Answer play_arrow
question_answer 171) Total number of all species of organisms in a given region is known as the regions
A)
biota
done
clear
B)
flora
done
clear
C)
fauna
done
clear
D)
diversity
done
clear
View Answer play_arrow
question_answer 172) The arthropod exoskeleton is composed of
A)
several kinds of polysaccharides
done
clear
B)
layers of proteins and a polysaccharide called chitin
done
clear
C)
several kinds of proteins
done
clear
D)
single complex protein called arthropodin
done
clear
View Answer play_arrow
question_answer 173) Which of the following groups is absolutely essential functional component of the ecosystem?
A)
Producers
done
clear
B)
Producers and herbivores
done
clear
C)
Producers and detritivores
done
clear
D)
Detritivores
done
clear
View Answer play_arrow
question_answer 174) Phagocytosis and pinocytosis are collectively termed as
A)
endocytosis
done
clear
B)
suspension feeding
done
clear
C)
omnivores
done
clear
D)
mucous trap
done
clear
View Answer play_arrow
question_answer 175) PCR proceeds in three distinct steps governed by temperature, they are in order of
A)
denaturation, annealing, synthesis
done
clear
B)
synthesis, annealing, denaturation
done
clear
C)
annealing, synthesis, denaturation
done
clear
D)
denaturation, synthesis, annealing
done
clear
View Answer play_arrow
question_answer 176) Corpus luteum releases
A)
oestrogen
done
clear
B)
progesterone
done
clear
C)
oestrogen and progesterone
done
clear
D)
androgen
done
clear
View Answer play_arrow
question_answer 177) Which of the following organs is devoid of glands?
A)
Uterus
done
clear
B)
Vagina
done
clear
C)
Vulva
done
clear
D)
Oviduct
done
clear
View Answer play_arrow
question_answer 178) Primary spermatocyte differs from spermatogonium in
A)
number of chromosomes
done
clear
B)
size and volume
done
clear
C)
DNA content
done
clear
D)
size of chromosomes
done
clear
View Answer play_arrow
question_answer 179) In human, cleavage divisions are
A)
slow and synchronous
done
clear
B)
fast and synchronous
done
clear
C)
slow and asynchronous
done
clear
D)
fast and asynchronous
done
clear
View Answer play_arrow
question_answer 180) The basic unit of study in Ecology is
A)
population
done
clear
B)
organism
done
clear
C)
community
done
clear
D)
species
done
clear
View Answer play_arrow
question_answer 181) Chimera is produced due to
A)
somatic mutations
done
clear
B)
reverse mutations
done
clear
C)
lethal mutations
done
clear
D)
pleiotropic mutations
done
clear
View Answer play_arrow
question_answer 182) Maltose gives rise to two molecules of
A)
fructose
done
clear
B)
lactose
done
clear
C)
glucose
done
clear
D)
sucrose
done
clear
View Answer play_arrow
question_answer 183) In a lake, phytoplankton grow in abundance in
A)
littoral zone
done
clear
B)
limnetic zone
done
clear
C)
profundal zone
done
clear
D)
benthic region
done
clear
View Answer play_arrow
question_answer 184) Sigmoid growth curve is represented by
A)
\[dN/dt=rN\]
done
clear
B)
\[dN/dt=rN\,\,(1-N/K)\]
done
clear
C)
\[Nt/No+B+I-D-E\]
done
clear
D)
\[dN/dt=1-N/K\]
done
clear
View Answer play_arrow
question_answer 185) Beadle and Tatum showed that each kind of mutant bread mould they studied lacked a specific enzyme. Their experiments demonstrated that
A)
cells need specific enzymes in order to function
done
clear
B)
genes are made of DNA
done
clear
C)
genes carry information for making proteins
done
clear
D)
enzymes are required to repair damaged DNA information
done
clear
View Answer play_arrow
question_answer 186) DNA has equal number of adenine an thymine residues (A = T) and equal number of guanine and cytosine (G = C). These relationships are known as
A)
Chargaff s rule
done
clear
B)
Coulombs law
done
clear
C)
Le-Chateliers principle
done
clear
D)
Vant Hoff plot
done
clear
View Answer play_arrow
question_answer 187) Balancing selection promotes
A)
hoirzygotes
done
clear
B)
heterozygotes
done
clear
C)
Polyploids
done
clear
D)
recessive traits
done
clear
View Answer play_arrow
question_answer 188) Vomiting centre is located in the
A)
medulla oblongata
done
clear
B)
stomach and sometimes in duodenum
done
clear
C)
GI tract
done
clear
D)
hypothalamus
done
clear
View Answer play_arrow
question_answer 189) How many bio-geographical regions are present in India?
A)
3
done
clear
B)
4
done
clear
C)
7
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 190) Vital stains are employed to study
A)
living cells
done
clear
B)
frozen tissues
done
clear
C)
fresh tissues
done
clear
D)
preserved tissues
done
clear
View Answer play_arrow
question_answer 191) Which of the following organs in earthworm neutralizes humic acid present in humus?
A)
Typhlosole
done
clear
B)
Calciferous glands
done
clear
C)
Intestinal caecum
done
clear
D)
Gizzard
done
clear
View Answer play_arrow
question_answer 192) Fertilized eggs of P. americana are encased in
A)
ootheca
done
clear
B)
cocoon
done
clear
C)
genital chamber
done
clear
D)
phallomere
done
clear
View Answer play_arrow
question_answer 193) Insufficient quantities of antidiuretic hormone in blood lead to
A)
diabetes mellitus
done
clear
B)
glycosuria
done
clear
C)
diabetes insipidus
done
clear
D)
uremia
done
clear
View Answer play_arrow
question_answer 194) Sphincter of Oddi guards
A)
hepato-pancreatic duct
done
clear
B)
common bile duct
done
clear
C)
pancreatic duct
done
clear
D)
cystic duct
done
clear
View Answer play_arrow
question_answer 195) Graveyard for RBCs is
A)
liver
done
clear
B)
spleen
done
clear
C)
kidney
done
clear
D)
lymph glands
done
clear
View Answer play_arrow
question_answer 196) Blood cells involved in inflammatory reactions are
A)
basophils
done
clear
B)
neuti-ophils
done
clear
C)
eosinophils
done
clear
D)
monocytes
done
clear
View Answer play_arrow
question_answer 197) To obtain a standard ECG, a patient is connected to the machine with three electrodes
A)
one to each wrist and to the left ankle
done
clear
B)
one to each ankle and to the left wrist
done
clear
C)
one to each wrist and to the left chest Region
done
clear
D)
one to each ankle and to the left chest region
done
clear
View Answer play_arrow
question_answer 198) The clavicle articulates with ...... of scapula.
A)
acromion process
done
clear
B)
glenoid cavity
done
clear
C)
acetabulum cavity
done
clear
D)
ball and socket joint
done
clear
View Answer play_arrow
question_answer 199) The age of pyramid with broad base indicates
A)
high percentage of young individuals
done
clear
B)
low percentage of young individuals
done
clear
C)
high percentage of old individuals
done
clear
D)
low percentage of old individuals
done
clear
View Answer play_arrow
question_answer 200) Thymosin hormone is secreted by
A)
thyroid gland
done
clear
B)
parathyroid gland
done
clear
C)
thymus gland
done
clear
D)
hypothalamus
done
clear
View Answer play_arrow
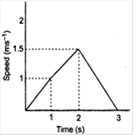
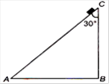
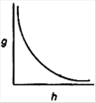
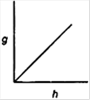
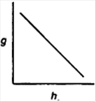

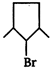
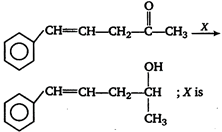
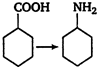 The sequence of the reagents used are
The sequence of the reagents used are
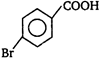
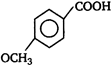
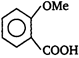 is stronger acid than
is stronger acid than