A) \[\log {{C}_{t}}\] vs time
B) \[\frac{1}{time}vs\,\,{{C}_{t}}\]
C) time \[vs\,\,{{C}_{t}}\]
D) \[\frac{1}{time}vs\frac{1}{{{C}_{t}}}\]
Correct Answer: A
Solution :
For first order reaction,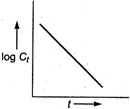 Integrated rate equation,\[kt=\log \,{{C}_{0}}-\log \,{{C}_{t}}\] The straight line graph is obtained by plotting \[\log \,{{C}_{t}}\]/ versus time.
Integrated rate equation,\[kt=\log \,{{C}_{0}}-\log \,{{C}_{t}}\] The straight line graph is obtained by plotting \[\log \,{{C}_{t}}\]/ versus time.
You need to login to perform this action.
You will be redirected in
3 sec
