question_answer 1) A black body at \[1227{}^\circ C\] emits radiations with maximum intensity at a wavelength of \[5000\overset{\text{o}}{\mathop{\text{A}}}\,\]. If the temperature of the body is increased by \[1000{}^\circ C\], the maximum intensity will be observed at
A)
\[4000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[5000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[6000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[3000\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 2) A transistor-oscillator using a resonant circuit with an inductor L (of negligible resistance) and a capacitor C in series produce oscillations of frequency\[f\]. If L is doubled and C is changed to 4C, the frequency will be
A)
\[f\]/4
done
clear
B)
8\[f\]
done
clear
C)
\[f\]/\[2\sqrt{2}\]
done
clear
D)
\[f\]/2
done
clear
View Answer play_arrow
question_answer 3) A Carnot engine whose sink is at 300 K has an efficiency of 40%. By how much should the temperature of source be increased so as to increase its efficiency by 50% of original efficiency?
A)
275 K
done
clear
B)
325 K
done
clear
C)
250 K
done
clear
D)
380 K
done
clear
View Answer play_arrow
question_answer 4) An \[\alpha \]-particle of energy 5 MeV is scattered through 180° by a fixed uranium nucleus. The distance of the closest approach is of the order of
A)
\[1\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[{{10}^{-10}}cm\]
done
clear
C)
\[{{10}^{-12}}cm\]
done
clear
D)
\[{{10}^{-15}}cm\]
done
clear
View Answer play_arrow
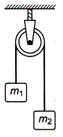
question_answer 5)
Two masses \[{{m}_{1}}=5kg\] and \[{{m}_{2}}=4.8\text{ }kg\] tied to a string are hanging over a light frictionless pulley. What is the acceleration of the masses when lift is free to move ? \[(g=9.8m/{{s}^{2}})\]
A)
\[0.2\text{ }m/{{s}^{2}}\]
done
clear
B)
\[9.8\text{ }m/{{s}^{2}}\]
done
clear
C)
\[5\text{ }m/{{s}^{2}}\]
done
clear
D)
\[4.8\text{ }m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 6) Alternating current cannot be measured by DC ammeter because
A)
AC cannot pass through DC ammeter
done
clear
B)
AC changes direction.
done
clear
C)
average value of current for complete ycle is zero
done
clear
D)
DC ammeter will get damaged
done
clear
View Answer play_arrow
question_answer 7) Two cells, having the same emf, are connected in series through an external resistance R. Cells have internal resistances \[{{r}_{1}}\]and \[{{r}_{2}}\]\[({{r}_{1}}>{{r}_{2}}),\] respectively. When the circuit is closed, the potential difference across the first cell is zero. The value of R is
A)
\[{{r}_{1}}-{{r}_{2}}\]
done
clear
B)
\[\frac{{{r}_{1}}+{{r}_{2}}}{2}\]
done
clear
C)
\[\frac{{{r}_{1}}+{{r}_{2}}}{2}\]
done
clear
D)
\[{{r}_{1}}+{{r}_{2}}\]
done
clear
View Answer play_arrow
question_answer 8) The binding energy of deuteron is 2.2 MeV and that of \[_{2}^{4}\]He is 28 MeV. If two deuterons are fused to form one \[_{2}^{4}\]He, then the energy released is
A)
25.8 MeV
done
clear
B)
23.6 MeV
done
clear
C)
19.2 MeV
done
clear
D)
30.2 MeV
done
clear
View Answer play_arrow
question_answer 9) lonization potential of hydrogen atom is 13.6 eV. Hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy 12.1 eV. According to Bohrs theory, the spectral lines emitted by hydrogen will be
A)
two
done
clear
B)
three
done
clear
C)
four
done
clear
D)
one
done
clear
View Answer play_arrow
question_answer 10) The potential energy of a long spring when stretched by 2 cm is \[U\]. If the spring is stretched by 8 cm the potential energy stored in it is
A)
4\[U\]
done
clear
B)
8\[U\]
done
clear
C)
16\[U\]
done
clear
D)
\[U\]/4
done
clear
View Answer play_arrow
question_answer 11) For angles of projection of a projectile at angles (45° - \[\text{ }\!\!\theta\!\!\text{ }\]) and (45° + \[\text{ }\!\!\theta\!\!\text{ }\]), the horizontal ranges described by the projectile are in the ratio of
A)
1 : 1
done
clear
B)
2 : 3
done
clear
C)
1: 2
done
clear
D)
2 :1
done
clear
View Answer play_arrow
question_answer 12) A body of mass 3 kg is under a constant force which causes a displacement s in metres in it, given by the relation \[s=\frac{1}{3}{{t}^{2}},\] where t is in 0 second. Work done by the force in 2 s is
A)
\[\frac{5}{19}J\]
done
clear
B)
\[\frac{3}{8}J\]
done
clear
C)
\[\frac{8}{3}J\]
done
clear
D)
\[\frac{19}{5}J\]
done
clear
View Answer play_arrow
question_answer 13) A particle moves along a straight line OX. At a time t (in second) the distance x (in metre) of the particle from 0 is given by \[x=40+12t-{{t}^{3}}\]how long would the particle travel before coming to rest?
A)
24 m
done
clear
B)
40 m
done
clear
C)
56 m
done
clear
D)
16 m
done
clear
View Answer play_arrow
question_answer 14) The velocity v of a particle at time t is given by \[v=at+\frac{b}{t+c}\]where a, b and c are constants. The dimensions of a, b and c are respectively
A)
\[[L{{T}^{2}}],[L]\] and \[[T]\]
done
clear
B)
\[[{{L}^{2}}],\text{ }\!\![\!\!\text{ T }\!\!]\!\!\text{ }\] and \[[L{{T}^{2}}]\]
done
clear
C)
\[[L{{T}_{2}}],[LT]\] and \[[L]\]
done
clear
D)
\[[L],[LT]\] and \[[{{T}^{2}}]\]
done
clear
View Answer play_arrow
question_answer 15) A microscope is focussed on a mark on a piece of paper and then a slab of glass of thickness 3 cm and refractive index 1.5 is placed over the mark. How should the microscope be moved to get the mark in focus again?
A)
1 cm upward
done
clear
B)
4.5 cm downward
done
clear
C)
1 cm downward
done
clear
D)
2 cm upward
done
clear
View Answer play_arrow
question_answer 16) 300 J of work is done in sliding a 2 kg block up an inclined plane of height 10 m. Taking \[g=10m/{{s}^{2}}\] work done against friction is
A)
200 J
done
clear
B)
100 J
done
clear
C)
zero
done
clear
D)
1000 J
done
clear
View Answer play_arrow
question_answer 17) A transistor is operated in common emitter configuration at constant collector voltage \[{{V}_{C}}=1-5V\] such that a change in the base current from 100 \[\mu \]A to 150 \[\mu \]A produces a change in the collector current from 5 mA to 10 mA. The current gain (\[\beta \]) is
A)
67
done
clear
B)
75
done
clear
C)
100
done
clear
D)
50
done
clear
View Answer play_arrow
question_answer 18) The core of a transformer is laminated because
A)
energy losses due to eddy currents may be minimized
done
clear
B)
the weight of the transformer may be reduced
done
clear
C)
rusting of the core may be prevented
done
clear
D)
ratio of voltage in primary and secondary may be increased
done
clear
View Answer play_arrow
question_answer 19) Two coils of self-inductances 2 mH and 8 mH are placed so close together that the effective flux in one coil is completely linked with the other. The mutual inductance between these coils is
A)
10 mH
done
clear
B)
6 mH
done
clear
C)
4 mH
done
clear
D)
16 mH
done
clear
View Answer play_arrow
question_answer 20) In a discharge tube ionization of enclosed gas is produced due to collisions between
A)
positive ions and neutral atoms/molecules
done
clear
B)
negative electrons and neutral atoms/molecules
done
clear
C)
photons and neutral atoms/molecules
done
clear
D)
neutral gas atoms/molecules
done
clear
View Answer play_arrow
question_answer 21) When photons of energy hv fall on an aluminium plate (of work function \[{{E}^{0}}\]), photoelectrons of maximum kinetic energy K are ejected. If the frequency of the radiation is doubled, the maximum kinetic energy of the ejected photoelectrons will be
A)
\[K+{{e}_{0}}\]
done
clear
B)
\[2K\]
done
clear
C)
\[K\]
done
clear
D)
\[K+hv\]
done
clear
View Answer play_arrow
question_answer 22) A coil of inductive reactance 31\[\Omega \] has a resistance of 8\[\Omega \]. It is placed in series with a condenser of capacitive reactance 25\[\Omega \]. The combination is connected to an AC source of 110 V. The power factor of the circuit is
A)
0.56
done
clear
B)
0.64
done
clear
C)
0.80
done
clear
D)
0.33
done
clear
View Answer play_arrow
question_answer 23) The moment of inertia of a uniform circular disc of radius R and mass M about an axis touching the disc at its diameter and normal to the disc is
A)
\[M{{R}^{2}}\]
done
clear
B)
\[\frac{2}{5}M{{R}^{2}}\]
done
clear
C)
\[\frac{3}{2}M{{R}^{2}}\]
done
clear
D)
\[\frac{1}{2}M{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 24) The momentum of a photon of energy 1 MeV in kg-m/s, will be
A)
\[0.33\times {{10}^{6}}\]
done
clear
B)
\[7\times {{10}^{-24}}\]
done
clear
C)
\[{{10}^{-22}}\]
done
clear
D)
\[5\times {{10}^{-22}}\]
done
clear
View Answer play_arrow
question_answer 25) The radius of germanium (Ge) nuclide is measured to be twice the radius of \[_{4}^{9}\]Be. The number of nucleons in Ge are
A)
73
done
clear
B)
74
done
clear
C)
75
done
clear
D)
72
done
clear
View Answer play_arrow
question_answer 26) The molar specific heat at constant pressure of an ideal gas is (7/2)R. The ratio of specific heat at constant pressure to that at constant volume is
A)
7/5
done
clear
B)
8/7
done
clear
C)
5/7
done
clear
D)
9/7
done
clear
View Answer play_arrow
question_answer 27) The earth is assumed to be a sphere of radius R.A platform is arranged at a height R from the surface of the earth. The escape velocity of a body from this platform is \[f{{v}_{e,}}\] where \[{{v}_{e}}\] is its escape velocity from the surface of the earth. The value of\[f\] is
A)
\[\sqrt{2}\]
done
clear
B)
\[\frac{1}{\sqrt{2}}\]
done
clear
C)
\[\frac{1}{3}\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 28) Two sound waves with wavelength 5.0 m and 5.5 m respectively, each propagate in a gas with velocity 330 m/s. We expect the following number of beats per second
A)
12
done
clear
B)
zero
done
clear
C)
1
done
clear
D)
6
done
clear
View Answer play_arrow
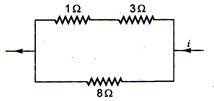
question_answer 29)
Power dissipated across the 8\[\Omega \] resistor in the circuit shown here is 2W. The power dissipated in watt units across the 3\[\Omega \] resistor is
A)
2.0
done
clear
B)
1.0
done
clear
C)
0.5
done
clear
D)
3.0
done
clear
View Answer play_arrow
question_answer 30) Kirchhoffs first and second laws for electrical circuits are consequences of
A)
conservation of energy
done
clear
B)
conservation of electric charge and energy respectively
done
clear
C)
conservation of electric charge
done
clear
D)
conservation of energy and electric charge respectively
done
clear
View Answer play_arrow
question_answer 31) A transverse wave propagating along x-axis is represented by \[y(x,t)=8.0\sin \left( 05\pi x-4\pi t-\frac{\pi }{4} \right)\]where x is in metre and t is in second. The speed of the wave is
A)
4\[\pi \] m/s
done
clear
B)
0.5 \[\pi \] m/s
done
clear
C)
\[\frac{\pi }{4}\] m/s
done
clear
D)
8 m/s
done
clear
View Answer play_arrow
question_answer 32) The time of reverberation of a room A is one second. What will be the time (in second) of reverberation of a room, having all the. dimensions double of those of room A?
A)
2
done
clear
B)
4
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 33) Which one of the following statements is true?
A)
Both light and sound waves in air are transverse
done
clear
B)
The sound waves in air are longitudinal while the light waves are transverse
done
clear
C)
Both light and sound waves in air are longitudinal
done
clear
D)
Both light and sound waves can travel in vacuum
done
clear
View Answer play_arrow
question_answer 34) Above Curie temperature
A)
a ferromagnetic substance becomes paramagnetic
done
clear
B)
a paramagnetic substance becomes diamagnetic
done
clear
C)
a diamagnetic substance becomes paramagnetic
done
clear
D)
a paramagnetic substance becomes ferromagnetic
done
clear
View Answer play_arrow
question_answer 35) A convex lens and a concave lens, each having same focal length of 25 cm, are put in contact to form a combination of lenses. The power in diopters of the combination is
A)
25
done
clear
B)
50
done
clear
C)
infinite
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 36) An electric dipole of moment \[\overrightarrow{\text{P}}\] is lying along a uniform electric field \[\overrightarrow{\text{E}\text{.}}\]The work done in rotating the dipole by 90° is
A)
\[\sqrt{2}pE\]
done
clear
B)
\[\frac{pE}{2}\]
done
clear
C)
\[2pE\]
done
clear
D)
\[pE\]
done
clear
View Answer play_arrow
question_answer 37) A car runs at a constant speed on a circular track of radius 100 m, taking 62.8 s for every circular lap. The average velocity and average speed for each circular lap respectively is
A)
0, 0
done
clear
B)
0, 10 m/s
done
clear
C)
10 m/s, 10 m/s
done
clear
D)
10 m/s, 0
done
clear
View Answer play_arrow
question_answer 38) A tube of length L is filled completely with an incompressible liquid of mass M and closed at both the ends. The tube is then rotated in a horizontal plane about one of its ends with a uniform angular velocity \[\omega .\]The force exerted by the liquid at the other end is
A)
\[\frac{ML{{\omega }^{2}}}{2}\]
done
clear
B)
\[\frac{M{{L}^{2}}{{\omega }^{{}}}}{2}\]
done
clear
C)
\[ML{{\omega }^{2}}\]
done
clear
D)
\[\frac{M{{L}^{2}}{{\omega }^{2}}}{2}\]
done
clear
View Answer play_arrow
question_answer 39) Which of the following statements is correct for any thermodynamic system?
A)
The internal energy changes in all processes
done
clear
B)
Internal energy and entropy are state functions
done
clear
C)
The change in entropy can never be zero
done
clear
D)
The work done in an adiabatic process is always zero
done
clear
View Answer play_arrow
question_answer 40) The vectors \[\text{\vec{A}}\] and \[\text{\vec{B}}\] are such that\[\text{ }\!\!|\!\!\text{ }\overrightarrow{\text{A}}\text{+}\overrightarrow{\text{B}}\text{ }\!\!|\!\!\text{ }\,\text{=}\,\text{ }\!\!|\!\!\text{ }\overrightarrow{\text{A}}\text{-}\overrightarrow{\text{B}}|\] The angle between the two vectors is
A)
\[90{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[75{}^\circ \]
done
clear
D)
\[45{}^\circ \]
done
clear
View Answer play_arrow
question_answer 41) Infrared radiations are detected by
A)
spectrometer
done
clear
B)
pyrometer
done
clear
C)
nanometer
done
clear
D)
photometer
done
clear
View Answer play_arrow
question_answer 42) Which of the following is more close to a black body?
A)
Black board paint
done
clear
B)
Green leaves
done
clear
C)
Black holes
done
clear
D)
Red roses
done
clear
View Answer play_arrow
question_answer 43) Formation of covalent bonds in compounds exhibits
A)
wave nature of electron
done
clear
B)
particle nature of electron
done
clear
C)
both wave and particle nature of electron
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 44) The radius of a spherical drop of water is 1 mm. If surface tension of water be \[70\times {{10}^{-3}}N/m,\]the pressure difference inside and outside the drop will be
A)
\[70\text{ }N/{{m}^{2}}\]
done
clear
B)
\[140\text{ }N/{{m}^{2}}\]
done
clear
C)
\[280\text{ }N/{{m}^{2}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 45) A laser beam is coherent because it contains
A)
waves of several wavelengths
done
clear
B)
incoherent waves of a single wavelength
done
clear
C)
coherent waves of several wavelengths
done
clear
D)
coherent waves of a single wavelength
done
clear
View Answer play_arrow
question_answer 46) Protein is a polymer of
A)
glucose
done
clear
B)
terephthalic acid
done
clear
C)
amino acid
done
clear
D)
glycol
done
clear
View Answer play_arrow
question_answer 47) \[HCl\] is completely neutralised by \[NaOH\] solution. The resulting solution contains the following species
A)
\[N{{a}^{+}},C{{l}^{-}},{{H}_{3}}{{O}^{+}}\]
done
clear
B)
\[N{{a}^{+}},C{{l}^{-}},{{H}_{2}}O\]
done
clear
C)
\[N{{a}^{+}},C{{l}^{-}},O{{H}^{-}}\]
done
clear
D)
\[{{H}^{+}},{{H}_{3}}{{O}^{+}}\]
done
clear
View Answer play_arrow
question_answer 48) The degree of dissociation of XY in case of following reaction is found to be \[0.4\] from vapour density measurement. If the observed vapour density of X Y be 100 then its molecular weight is \[XY\to X+Y\]
A)
\[140\]
done
clear
B)
\[70\]
done
clear
C)
\[280\]
done
clear
D)
\[210\]
done
clear
View Answer play_arrow
question_answer 49) For the reaction,\[C(s)+C{{O}_{2}}(g)2CO(g)\]the partial pressure of \[C{{O}_{2}}\] and \[CO\] are 2.0 and 4.0 arm respectively then equilibrium \[{{K}_{p}}\] for the reaction will be
A)
\[0.5\]
done
clear
B)
\[8.0\]
done
clear
C)
\[4.0\]
done
clear
D)
\[32\]
done
clear
View Answer play_arrow
question_answer 50) Solubility of \[AgCl\] will be minimum in
A)
\[0.01M\text{ }NaCl\]solution
done
clear
B)
\[0.01M\text{ }CaC{{l}_{2}}\] solution
done
clear
C)
pure water
done
clear
D)
\[0.001M\,\,\,AgN{{O}_{3}}\]solution
done
clear
View Answer play_arrow
question_answer 51) pH of \[HCl\] (cone.\[0.1mol/L\]) is one. Compute the pH of 0.05 mol/L solution of \[{{H}_{2}}S{{O}_{4}}\].
A)
\[0.05\]
done
clear
B)
\[0.5\]
done
clear
C)
\[1\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 52) What is the pH at equivalence point in the titration of \[0.1M\,\,\,C{{H}_{3}}COOH\]and \[0.1M\]\[NaOH\]? (\[{{K}_{a}}\] for acetic acid is\[1.8\times {{10}^{-5}}\])
A)
7
done
clear
B)
Between 7 and 8
done
clear
C)
Between 8 and 9
done
clear
D)
Between 6 and 7
done
clear
View Answer play_arrow
question_answer 53)
Given Acid \[p{{K}_{a}}\] \[C{{H}_{3}}C{{O}_{2}}H\] (i) \[0.7\] \[CC{{l}_{3}}C{{O}_{2}}H\] (ii) \[0.2\] \[ClC{{H}_{2}}C{{O}_{2}}H\] (iii) \[4.8\] \[{{F}_{3}}CC{{O}_{2}}H\] (iv) \[2.9\]
The correct code is
A)
A-(iii) B- (i) C-(iv) D- (ii)
done
clear
B)
A- (ii) B-(iv) C-(i) D- (iii)
done
clear
C)
A- (iii) B- (ii) C-(i) D- (iv)
done
clear
D)
A-(i) B-(iii) C-(ii) D- (iv)
done
clear
View Answer play_arrow
question_answer 54) A basic buffer solution is formed by selecting the pair
A)
\[KCN-KOH\]
done
clear
B)
\[C{{H}_{3}}COOH-NaCl\]
done
clear
C)
\[N{{H}_{4}}OH-C{{H}_{3}}COON{{H}_{4}}\]
done
clear
D)
\[NaCI-NaOH\]
done
clear
View Answer play_arrow
question_answer 55) Which will have the minimum value of \[p{{K}_{b}}\]?
A)
\[O{{H}^{-}}\]
done
clear
B)
\[C{{H}_{3}}CO{{O}^{-}}\]
done
clear
C)
\[Cr\]
done
clear
D)
\[HCO{{O}^{-}}\]
done
clear
View Answer play_arrow
question_answer 56) A first order reaction complete ifs \[10%\] in 20 min then time required to complete-its \[19%\]is (log\[3=0.477\])
A)
\[30\text{ }min\]
done
clear
B)
\[40min\]
done
clear
C)
\[38min\]
done
clear
D)
\[45min\]
done
clear
View Answer play_arrow
question_answer 57) The \[\Delta H\] value of the reaction,\[{{H}_{2}}+C{{l}_{2}}2HCl\] is \[-44.2kcal\] If \[{{E}_{1}}\] is the activation energy of the forward reaction, then for the above reaction activation energy of reverse reaction \[{{E}_{2}}\] is
A)
\[{{E}_{1}}>{{E}_{2}}\]
done
clear
B)
\[{{E}_{1}}<{{E}_{2}}\]
done
clear
C)
\[{{E}_{1}}={{E}_{2}}\]
done
clear
D)
\[\Delta H\] is not related to \[{{E}_{1}}\] and \[{{E}_{2}}\]
done
clear
View Answer play_arrow
question_answer 58) The integrated rate equation is \[kt=\log \,{{C}_{0}}-\log {{C}_{t}}\]. The straight line graph is obtained by plotting
A)
\[\log {{C}_{t}}\] vs time
done
clear
B)
\[\frac{1}{time}vs\,\,{{C}_{t}}\]
done
clear
C)
time \[vs\,\,{{C}_{t}}\]
done
clear
D)
\[\frac{1}{time}vs\frac{1}{{{C}_{t}}}\]
done
clear
View Answer play_arrow
question_answer 59) A mixture has \[18g\]water and \[414g\]ethanol. The mole fraction of water in mixture is (assume ideal behaviour of the mixture)
A)
\[0.1\]
done
clear
B)
\[0.4\]
done
clear
C)
\[0.7\]
done
clear
D)
\[0.9\]
done
clear
View Answer play_arrow
question_answer 60) An aqueous solution of methanol in water has vapour pressure
A)
equal to that of water
done
clear
B)
equal to that of methanol
done
clear
C)
more than that of water
done
clear
D)
less than that of water
done
clear
View Answer play_arrow
question_answer 61) Which of the following liquid pairs shows a positive deviation from Raoults law?
A)
Water-nitric acid
done
clear
B)
Benzene-methanol
done
clear
C)
Water-hydrochloric acid
done
clear
D)
Acetone-chloroform
done
clear
View Answer play_arrow
question_answer 62) A solution of sucrose (molar mass\[=342\text{ }g/mol\]) is prepared by dissolving 68.4 g of it per litre of the solution, what is its osmotic pressure? (\[R=0.082L\text{ }atm\text{ }{{K}^{-1}}\text{ m}o{{l}^{-1}}\]) at 273 K
A)
\[6.02\text{ }atm\]
done
clear
B)
\[4.477atm\]
done
clear
C)
\[3.4atm\]
done
clear
D)
\[5.32atm\]
done
clear
View Answer play_arrow
question_answer 63) How many atoms of calcium will be deposited from a solution of \[CaC{{l}_{2}}\]by a current of 25 milliamperes flowing for 60 s
A)
\[4.68\times {{10}^{18}}\]
done
clear
B)
\[4.68\times {{10}^{15}}\]
done
clear
C)
\[4.68\times {{10}^{12}}\]
done
clear
D)
\[4.68\times {{10}^{9}}\]
done
clear
View Answer play_arrow
question_answer 64) EMF of a cell whose half cells are given below is \[M{{g}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Mg(s);\] \[E=-2.37V\] \[C{{u}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Cu(s);\] \[E=+0.33V\]
A)
\[-2.03V\]
done
clear
B)
\[1.36V\]
done
clear
C)
\[2.7V\]
done
clear
D)
\[2.03V\]
done
clear
View Answer play_arrow
question_answer 65) Which of the following does not give a white precipitate with \[AgN{{O}_{3}}\]solution?
A)
Propyne
done
clear
B)
1-butyne
done
clear
C)
2-butyne
done
clear
D)
1-pentyne
done
clear
View Answer play_arrow
question_answer 66) Electrode potentials of five elements A, B, C, D and E are respectively \[-1.36,-0.32,0,-1.26\] and \[-6.42\]. The reactivity order of these elements are in the decreasing order of
A)
A, D, E, B and C
done
clear
B)
C, B, E, D and A
done
clear
C)
B, D, E, A and C
done
clear
D)
C, A, E, D and B
done
clear
View Answer play_arrow
question_answer 67) Which does not have carboxylic group?
A)
Benzoic acid
done
clear
B)
Lactic acid
done
clear
C)
Picric acid
done
clear
D)
Aspirin
done
clear
View Answer play_arrow
question_answer 68) Which of the following has highest chlorine content?
A)
Pyrene
done
clear
B)
DDT
done
clear
C)
Chloral
done
clear
D)
BHC
done
clear
View Answer play_arrow
question_answer 69) Acetamide and \[NaOBr/O{{H}^{-}}\] produce
A)
ethanamide
done
clear
B)
methanamine
done
clear
C)
ethanamine
done
clear
D)
formic acid
done
clear
View Answer play_arrow
question_answer 70) Vitamin \[{{B}_{12}}\]contains
A)
\[Co(III)\]
done
clear
B)
\[Zn(II)\]
done
clear
C)
v
done
clear
D)
\[Fe(II)\]
done
clear
View Answer play_arrow
question_answer 71) Which of the following is maximum basic?
A)
\[{{({{C}_{2}}{{H}_{5}})}_{2}}NH\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}NH\]
done
clear
C)
\[{{({{C}_{2}}{{H}_{5}})}_{3}}N\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 72) Calcium carbide on hydrolysis form
A)
ethane
done
clear
B)
ethylene
done
clear
C)
methane
done
clear
D)
acetylene
done
clear
View Answer play_arrow
question_answer 73) Collins reagent is
A)
\[Mn{{O}_{2}}/HCl\]
done
clear
B)
\[Mn{{O}_{4}}/{{C}_{5}}{{H}_{5}}N\]
done
clear
C)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[C{{r}_{2}}{{O}_{3}}/2{{C}_{5}}{{H}_{5}}N\]
done
clear
View Answer play_arrow
question_answer 74) General formula of paraffin is
A)
\[{{C}_{n}}{{H}_{2n}}\]
done
clear
B)
\[{{C}_{n}}{{H}_{2n-2}}\]
done
clear
C)
\[{{C}_{n}}{{H}_{2n+2}}\]
done
clear
D)
\[{{C}_{2n}}{{H}_{2n}}\]
done
clear
View Answer play_arrow
question_answer 75) Which of the following does not give iodoform reaction?
A)
\[PhCOC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
C)
\[C{{H}_{3}}OH\]
done
clear
D)
\[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 76) The reaction,\[C{{H}_{2}}=CHC{{H}_{3}}+HBr\xrightarrow{{}}C{{H}_{3}}CHBrC{{H}_{3}}\]is a type of
A)
electrophilic addition reaction
done
clear
B)
nucleophilic addition reaction
done
clear
C)
free radical addition reaction
done
clear
D)
electrophilic substitution reaction
done
clear
View Answer play_arrow
question_answer 77) Which of the following aldehyde is most reactive towards nucleophilic addition reaction?
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}PHO\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}CHO\]
done
clear
View Answer play_arrow
question_answer 78) An. unknown compound has molar mass\[240g/mol\] . Its empirical formula is \[C{{H}_{2}}O\] then its molecular formula will be
A)
\[{{C}_{4}}{{H}_{8}}{{O}_{4}}\]
done
clear
B)
\[{{C}_{12}}{{H}_{22}}{{O}_{12}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\]
done
clear
D)
\[{{C}_{8}}{{H}_{16}}{{O}_{8}}\]
done
clear
View Answer play_arrow
question_answer 79) Which of the following give \[{{H}_{2}}\] gas with Na?
A)
\[C{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 80) Phenol when treated with phthalic anhydride in presence of cone. \[{{H}_{2}}S{{O}_{4}}\] forms X which produces pink colour on reaction with base. The compound X is
A)
benzene sulphonic acid
done
clear
B)
Phenolphthalene
done
clear
C)
salicylic acid
done
clear
D)
nitrophenol
done
clear
View Answer play_arrow
question_answer 81) Blue vitriol has
A)
ionic bond
done
clear
B)
hydrogen bond
done
clear
C)
coordinate bond
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 82) According to MOT, \[{{C}_{2}}\] molecule has
A)
one a and one n bond
done
clear
B)
only two n bonds
done
clear
C)
only two a bonds
done
clear
D)
one o and two n bonds
done
clear
View Answer play_arrow
question_answer 83) What is wrong about \[CO_{3}^{2-}\] ion ?
A)
It has planar structure
done
clear
B)
It has one coordinate bond
done
clear
C)
It has three resonating structures
done
clear
D)
Hydrolysis of \[CO_{3}^{2-}\] ion gives basic solution
done
clear
View Answer play_arrow
question_answer 84) Which of the following is most polar?
A)
\[C{{H}_{3}}Cl\]
done
clear
B)
\[C{{H}_{3}}F\]
done
clear
C)
\[C{{H}_{3}}Br\]
done
clear
D)
\[C{{H}_{3}}I\]
done
clear
View Answer play_arrow
question_answer 85) Which of the following will furnish \[F{{e}^{3+}}\] ions in solution?
A)
\[F{{e}_{4}}{{[Fe{{(CN)}_{6}}]}_{3}}\]
done
clear
B)
\[{{(N{{H}_{4}})}_{2}}S{{O}_{4}}.FeS{{O}_{4}}.6{{H}_{2}}O\]
done
clear
C)
\[F{{e}_{3}}{{[Fe{{(CN)}_{6}}]}_{2}}\]
done
clear
D)
\[{{[Fe{{(CN)}_{6}}]}^{3-}}\]
done
clear
View Answer play_arrow
question_answer 86) When \[KMn{{O}_{4}}\]reacts with acidified \[FeS{{O}_{4}}\]
A)
only \[FeS{{O}_{4}}\] is oxidized
done
clear
B)
only \[KMn{{O}_{4}}\] is oxidized
done
clear
C)
\[FeS{{O}_{4}}\] is oxidised and \[KMn{{O}_{4}}\] is reduced
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 87) Which of the following electronic configuration corresponds to the highest value of ionisation potential?
A)
\[[Xe]6{{s}^{2}}\]
done
clear
B)
\[[Ar]4{{s}^{2}}3{{d}^{10}}\]
done
clear
C)
\[1{{s}^{2}}\]
done
clear
D)
\[[Rn]7{{s}^{2}}6{{d}^{1}}5{{f}^{14}}\]
done
clear
View Answer play_arrow
question_answer 88) An element with atomic number 112 has been made recently. It should be
A)
an actinide
done
clear
B)
a transition metal
done
clear
C)
a noble gas
done
clear
D)
a lanthanide
done
clear
View Answer play_arrow
question_answer 89) Product of the following reaction is\[A{{l}_{4}}{{C}_{3}}+{{D}_{2}}O\xrightarrow{{}}\]
A)
\[Al{{(OD)}_{3}}+C{{D}_{4}}\]
done
clear
B)
\[Al{{(OD)}_{2}}+C{{D}_{4}}\]
done
clear
C)
\[Al{{(OD)}_{4}}+C{{D}_{4}}\]
done
clear
D)
\[Al{{(OD)}_{3}}+CD\]
done
clear
View Answer play_arrow
question_answer 90) Which is maximum basic?
A)
\[N{{F}_{3}}\]
done
clear
B)
\[NC{{l}_{3}}\]
done
clear
C)
\[NB{{r}_{3}}\]
done
clear
D)
\[N{{I}_{3}}\]
done
clear
View Answer play_arrow
question_answer 91) Which is not a protein?
A)
Alpha amylase
done
clear
B)
Ribozyme
done
clear
C)
Histidine kinase
done
clear
D)
Nitrogenase
done
clear
View Answer play_arrow
question_answer 92) Black pepper is
A)
herb
done
clear
B)
shrub
done
clear
C)
tree
done
clear
D)
climber
done
clear
View Answer play_arrow
question_answer 93) Which of the followings is not caused by deficiency of mineral ?
A)
Chlorosis
done
clear
B)
Etiolation
done
clear
C)
Shortening of intemodes
done
clear
D)
Necrosis
done
clear
View Answer play_arrow
question_answer 94) Retrovirus have genetic material
A)
DNA only
done
clear
B)
RNA only
done
clear
C)
DNA or RNA only
done
clear
D)
either DNA or RNA only
done
clear
View Answer play_arrow
question_answer 95) In Funaria calyptra arises from
A)
capsule
done
clear
B)
antheridium
done
clear
C)
columella
done
clear
D)
archegonium
done
clear
View Answer play_arrow
question_answer 96) DCMU
A)
inhibits PS-I
done
clear
B)
inhibits PS-II
done
clear
C)
destroy chloroplast
done
clear
D)
inhibits oxidative phosphoryladon
done
clear
View Answer play_arrow
question_answer 97) Prions consist mainly of
A)
protein
done
clear
B)
DNA
done
clear
C)
RNA
done
clear
D)
Both (b) and (c)
done
clear
View Answer play_arrow
question_answer 98) \[N{{H}_{3}}\]releases from
A)
photorespiration
done
clear
B)
dark respiration
done
clear
C)
CAM
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 99) According to Kyoto protocol the major nations abide to reduce concentration of greenhouse gases by.
A)
2008
done
clear
B)
2010
done
clear
C)
2012
done
clear
D)
2018
done
clear
View Answer play_arrow
question_answer 100) Hela cells used in cell biology are
A)
cancerous cells grown in cancer research laboratory
done
clear
B)
cervical cancer cell derivatives
done
clear
C)
Both (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 101) In 2002 AD according to research the concentration of CFC reached to
A)
368 ppm
done
clear
B)
1750 ppb
done
clear
C)
261 ppt
done
clear
D)
326 ppb
done
clear
View Answer play_arrow
question_answer 102) Which of the following causes abortion in ladies?
A)
Viruses
done
clear
B)
Bacteria
done
clear
C)
Mycoplasma
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 103) Which of the following is a prokaryote?
A)
Amoeba
done
clear
B)
Spirogyra
done
clear
C)
Bacteria
done
clear
D)
Chlamydomonas
done
clear
View Answer play_arrow
question_answer 104) Ginger is an underground stem. It is distinguished from root because
A)
it lacks chlorophyll
done
clear
B)
it stores food
done
clear
C)
it has nodes and intemodes
done
clear
D)
it has xylem and vessels
done
clear
View Answer play_arrow
question_answer 105) DNA replication includes
A)
DNA ligase
done
clear
B)
DNA polymerase and ligase
done
clear
C)
RNA polymerase
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 106) During DNA replication in prokaryotes DNA is anchored to
A)
chromosome
done
clear
B)
mesosome
done
clear
C)
nucleolus
done
clear
D)
ribosome
done
clear
View Answer play_arrow
question_answer 107) Stain used by Feulgen to stain DNA is
A)
janus green
done
clear
B)
basic fuschin
done
clear
C)
crystal violet
done
clear
D)
methylene blue
done
clear
View Answer play_arrow
question_answer 108) The mineral present in cell wall is
A)
Na
done
clear
B)
Ca
done
clear
C)
K
done
clear
D)
Mg
done
clear
View Answer play_arrow
question_answer 109) Male gamete in angiosperm is produced by
A)
Generative cell
done
clear
B)
Microspore cell
done
clear
C)
Vegetative cell
done
clear
D)
Tube cell
done
clear
View Answer play_arrow
question_answer 110) Meristematic tissue in vascular bundle is
A)
phellem
done
clear
B)
procambium
done
clear
C)
interfasicular cambium
done
clear
D)
fasicular cambium
done
clear
View Answer play_arrow
question_answer 111) Study of Ecology of population is called
A)
Autecology
done
clear
B)
Synecology
done
clear
C)
Ecotype
done
clear
D)
Demecology
done
clear
View Answer play_arrow
question_answer 112) Dwarf wheat was developed by
A)
M S Swaminathan
done
clear
B)
Vavilov
done
clear
C)
Borlaug
done
clear
D)
B D Singh
done
clear
View Answer play_arrow
question_answer 113) A pure tall and a pure dwarf plant were crossed to produce offsprings. Offsprings were self-crossed, then find out the ratio between true breeding tall to true breeding dwarf ?
A)
1 : 1
done
clear
B)
3 :1
done
clear
C)
2 : 1
done
clear
D)
1 : 2 : 1
done
clear
View Answer play_arrow
question_answer 114) Which of the following plant is LDP ?
A)
Xanthium
done
clear
B)
Soybean
done
clear
C)
Wheat
done
clear
D)
Tobacco
done
clear
View Answer play_arrow
question_answer 115) Genophore term was coined by Hans R is for
A)
genetic material of virus
done
clear
B)
stack on which spore originated
done
clear
C)
bacterial chromosome
done
clear
D)
fungal chromosome
done
clear
View Answer play_arrow
question_answer 116) Cross between unrelated group of organisms is called
A)
hybrid
done
clear
B)
test cross
done
clear
C)
back cross
done
clear
D)
(d hleterosis
done
clear
View Answer play_arrow
question_answer 117) Which of the following is used as a best genetic vector in plants?
A)
Bacillus thurengiensis
done
clear
B)
Agrobacterium tumifaciens
done
clear
C)
Pseudomonas putida
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 118) Genetics term was proposed by
A)
Mendel
done
clear
B)
Bateson
done
clear
C)
Morgan
done
clear
D)
Johannson
done
clear
View Answer play_arrow
question_answer 119) One gene-one enzyme hypothesis of Beadle and Tatum was experimentally proved on,
A)
Saccaromyces
done
clear
B)
Neurospora crassa
done
clear
C)
Lathyrus odoratus
done
clear
D)
Claviceps
done
clear
View Answer play_arrow
question_answer 120) Cytochrome oxidase is a/an
A)
exoenzyme
done
clear
B)
endoenzyme
done
clear
C)
proenzyme
done
clear
D)
coenzyme
done
clear
View Answer play_arrow
question_answer 121) Which of the following is not a pyrimidine ?
A)
Thymine
done
clear
B)
Uracil
done
clear
C)
Guanine
done
clear
D)
Cytosine
done
clear
View Answer play_arrow
question_answer 122) Fusiform initial forms
A)
vascular rays
done
clear
B)
ray parenchyma
done
clear
C)
tracheary elements
done
clear
D)
primary phloem
done
clear
View Answer play_arrow
question_answer 123) Which organelle is present in higher number in secretory cells?
A)
Dictyosome
done
clear
B)
ER
done
clear
C)
Lysosome
done
clear
D)
Vacuole
done
clear
View Answer play_arrow
question_answer 124) Bio-indicators are used for
A)
oxygen demand
done
clear
B)
air pollution
done
clear
C)
mineral present
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 125) Phototropism is due to the hormone
A)
IAA
done
clear
B)
GA
done
clear
C)
2, 4-D
done
clear
D)
cytokinin
done
clear
View Answer play_arrow
question_answer 126) DNA is acidic due to
A)
sugar
done
clear
B)
phosphoric acid
done
clear
C)
purine
done
clear
D)
pyrimidine
done
clear
View Answer play_arrow
question_answer 127) Highest number of enzymes is found in
A)
lysosome
done
clear
B)
chloroplast
done
clear
C)
mitochondria
done
clear
D)
peroxisome
done
clear
View Answer play_arrow
question_answer 128) Psammophytes are plants that grow where soil is
A)
alkaline
done
clear
B)
sandy
done
clear
C)
acidic
done
clear
D)
alluvial
done
clear
View Answer play_arrow
question_answer 129) Nitrifying bacteria are able to
A)
convert atmospheric nitrogen into soluble forms
done
clear
B)
convert ammonia to nitrate
done
clear
C)
ammonia to nitrogen
done
clear
D)
nitrate to nitrogen
done
clear
View Answer play_arrow
question_answer 130) Huxley is father of
A)
classical taxonomy
done
clear
B)
artificial taxonomy
done
clear
C)
neo-taxonomy
done
clear
D)
adansonian taxonomy
done
clear
View Answer play_arrow
question_answer 131) RNA is not found in
A)
chromosome
done
clear
B)
plasmalemma
done
clear
C)
nucleolus
done
clear
D)
ribosome
done
clear
View Answer play_arrow
question_answer 132) Systema Naturae is written by
A)
Linnaeus
done
clear
B)
Aristotle
done
clear
C)
Hippocrates
done
clear
D)
Darwin
done
clear
View Answer play_arrow
question_answer 133) Binomial nomenclature was first issued in
A)
Systema Naturae
done
clear
B)
Genera Plantarum
done
clear
C)
Genera Animalium
done
clear
D)
Historia Plantarum
done
clear
View Answer play_arrow
question_answer 134) Number of chromosome in an angiospermic plant is 14, then the number of chromosome in synergid cells will be
A)
14
done
clear
B)
7
done
clear
C)
23
done
clear
D)
21
done
clear
View Answer play_arrow
question_answer 135) In September 2001, which of the following was used as a bioweapon agent in America ?
A)
botulinum
done
clear
B)
anthrax (Bacillus anthracis)
done
clear
C)
polio virus
done
clear
D)
AIDS virus
done
clear
View Answer play_arrow
question_answer 136) Formation of RNA on DNA template is called
A)
transduction
done
clear
B)
transformation
done
clear
C)
transcription
done
clear
D)
transfer
done
clear
View Answer play_arrow
question_answer 137) Presence of tail in a child is an example of
A)
atavism
done
clear
B)
divergent evolution
done
clear
C)
convergent evolution
done
clear
D)
mutation
done
clear
View Answer play_arrow
question_answer 138) To confirm ELISA for AIDS we used
A)
western blotting
done
clear
B)
northern blotting
done
clear
C)
southern blotting
done
clear
D)
eastern blotting
done
clear
View Answer play_arrow
question_answer 139) Action potential is generated by
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 140) Hot dilute soup was given by
A)
Oparin
done
clear
B)
Haldane
done
clear
C)
Urey
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 141) Aqueous and vitreous humour are divided by
A)
lens
done
clear
B)
iris
done
clear
C)
retina
done
clear
D)
optic nerve
done
clear
View Answer play_arrow
question_answer 142) Gene for colourblindness is located on
A)
Y chromosome
done
clear
B)
13th chromosome
done
clear
C)
X chromosome
done
clear
D)
21st chromosome
done
clear
View Answer play_arrow
question_answer 143) Species going to extinct due to low reproductive rate is
A)
lion
done
clear
B)
bald eagle
done
clear
C)
giant panda
done
clear
D)
island sp.
done
clear
View Answer play_arrow
question_answer 144) Indian rhinoceros are protected in
A)
Gir Forest
done
clear
B)
Kaziranga National Park
done
clear
C)
Bandipur National Park
done
clear
D)
Ranthambore National Park
done
clear
View Answer play_arrow
question_answer 145) Mitotic spindle have main protein
A)
tubulin
done
clear
B)
myosin
done
clear
C)
tropomyocin
done
clear
D)
dynein
done
clear
View Answer play_arrow
question_answer 146) Adrenal gland is derived from
A)
ectoderm
done
clear
B)
mesoderm
done
clear
C)
ectoderm and mesoderm
done
clear
D)
ectoderm and endoderm
done
clear
View Answer play_arrow
question_answer 147) Accessory sexual character in female is promoted by
A)
androgen
done
clear
B)
progesterone
done
clear
C)
estrogen
done
clear
D)
testosterone
done
clear
View Answer play_arrow
question_answer 148) X-lipked recessive gene is
A)
always expressed in male
done
clear
B)
always expressed in female
done
clear
C)
lethal
done
clear
D)
sub lethal
done
clear
View Answer play_arrow
question_answer 149) Hbisa
A)
reproductive pigment
done
clear
B)
respiratory pigment
done
clear
C)
carbohydrate
done
clear
D)
fat
done
clear
View Answer play_arrow
question_answer 150) Bile secretion is proportional to the concentration of
A)
protein
done
clear
B)
fat
done
clear
C)
carbohydrate
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 151) Ligament is mainly made up of
A)
reticulin
done
clear
B)
elastin
done
clear
C)
myosin
done
clear
D)
collagen
done
clear
View Answer play_arrow
question_answer 152) Secretion of pancreatic juice is stimulated by
A)
gastrin
done
clear
B)
secretin
done
clear
C)
enterogasteron
done
clear
D)
enterokinase
done
clear
View Answer play_arrow
question_answer 153) Out of 64 codons only 61 codes for the 20 different amino acids. This character of genetic code is called
A)
degeneracy
done
clear
B)
non ambiguous nature
done
clear
C)
redundancy
done
clear
D)
overlapping
done
clear
View Answer play_arrow
question_answer 154) Crossing over occurs in
A)
zygotene
done
clear
B)
leptotene
done
clear
C)
pachytene
done
clear
D)
diplotene
done
clear
View Answer play_arrow
question_answer 155) Connection between axon and dendrite is
A)
synapse
done
clear
B)
synapsis
done
clear
C)
desmosome
done
clear
D)
tight junction
done
clear
View Answer play_arrow
question_answer 156) Irregular nuclei is present in
A)
neutrophils
done
clear
B)
basophils
done
clear
C)
eosinophils
done
clear
D)
monocytes
done
clear
View Answer play_arrow
question_answer 157) Mesozoic era is golden period of
A)
reptiles
done
clear
B)
Mollusca
done
clear
C)
fishes
done
clear
D)
amphibians
done
clear
View Answer play_arrow
question_answer 158) Fossil found in Mandia district of MP is
A)
260 million years old
done
clear
B)
100 million years old
done
clear
C)
50 million years old
done
clear
D)
20 million years old
done
clear
View Answer play_arrow
question_answer 159) Which hormone is responsible for milk ejection after the birth of the baby ?
A)
Oxytocin
done
clear
B)
Progesterone
done
clear
C)
Prolactin
done
clear
D)
Estrogen
done
clear
View Answer play_arrow
question_answer 160) Hamburger shift is also known as
A)
bicarbonate shift
done
clear
B)
chloride shift
done
clear
C)
potassium shift
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 161) Bundle of His are
A)
nervous tissue supplied to ventricles
done
clear
B)
nervous tissue supplied to heart
done
clear
C)
muscular tissue supplied to ventricle
done
clear
D)
muscular tissue supplied to heart
done
clear
View Answer play_arrow
question_answer 162) Number of bones in skull is
A)
26
done
clear
B)
28
done
clear
C)
107
done
clear
D)
29
done
clear
View Answer play_arrow
question_answer 163) Cell theory was proposed by
A)
Virchow
done
clear
B)
Schleiden and Schwann
done
clear
C)
Robert Hooke
done
clear
D)
B McClintock
done
clear
View Answer play_arrow
question_answer 164) Viroids cause
A)
tobacco mosaic
done
clear
B)
tulip yellow mosaic
done
clear
C)
cauliflower mosaic
done
clear
D)
potato spindle mosaic
done
clear
View Answer play_arrow
question_answer 165) Who for the first time developed electron microscope?
A)
Knoll and Ruska
done
clear
B)
Rudolf and Kolliker
done
clear
C)
Robert Hooke
done
clear
D)
Swanson
done
clear
View Answer play_arrow
question_answer 166) Represser protein is produced by
A)
regulator gene
done
clear
B)
operator gene
done
clear
C)
structural gene
done
clear
D)
promoter gene
done
clear
View Answer play_arrow
question_answer 167) Nucleated RBC is found in
A)
man
done
clear
B)
rat
done
clear
C)
rabbit
done
clear
D)
frog
done
clear
View Answer play_arrow
question_answer 168) We know that the thyroxine controls metabolism in body. An autoimmune disease where the bodys own antibodies attack the cells of the thyroid is called
A)
Hyperthyroidism
done
clear
B)
Hashimotos disease
done
clear
C)
Graves disease
done
clear
D)
Turner syndrome
done
clear
View Answer play_arrow
question_answer 169) Acid hydrolase is found in
A)
Golgi body
done
clear
B)
ER
done
clear
C)
Lysosome
done
clear
D)
Vacuole
done
clear
View Answer play_arrow
question_answer 170) Autonomic nervous system effects on
A)
reflex actions
done
clear
B)
sensory organs
done
clear
C)
internal organ
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 171) Schizogony occurs in
A)
RBC of human
done
clear
B)
intestine of parasite
done
clear
C)
liver of human
done
clear
D)
liver of parasite
done
clear
View Answer play_arrow
question_answer 172) Haemophilia is due to absence of
A)
factor-VI
done
clear
B)
factor-VII
done
clear
C)
factor-VIII
done
clear
D)
factor-IX
done
clear
View Answer play_arrow
question_answer 173) Which of these is a dominant factor ?
A)
Rh factors
done
clear
B)
Haemophilia
done
clear
C)
Albinism
done
clear
D)
Colour blindness
done
clear
View Answer play_arrow
question_answer 174) Sertoli cells are found in testis. These cells are
A)
nurse cell
done
clear
B)
reproductive cell
done
clear
C)
receptor cell
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 175) Hinge joint is present between
A)
femur and ulna
done
clear
B)
humerus and ulna
done
clear
C)
femur and pectoral girdle
done
clear
D)
femur and pelvic girdle
done
clear
View Answer play_arrow
question_answer 176) Which is immortal ?
A)
Plasma cell
done
clear
B)
Germ cell
done
clear
C)
Brain cell
done
clear
D)
Kidney cell
done
clear
View Answer play_arrow
question_answer 177) After a deep inspiration and maximum expiration, the capacity of lungs is known as
A)
vital capacity
done
clear
B)
tidal volume
done
clear
C)
IRV
done
clear
D)
ERV
done
clear
View Answer play_arrow
question_answer 178) Cryptorchidism is a condition in which
A)
testis does not descend into scrotal sac
done
clear
B)
sperm is not found
done
clear
C)
male hormones are not reactive
done
clear
D)
ovaries are removed
done
clear
View Answer play_arrow
question_answer 179) Which one is a laevorotatory sugar ?
A)
Sucrose
done
clear
B)
Glucose
done
clear
C)
Fructose
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 180) Which one is the sweetest sugar?
A)
Glucose
done
clear
B)
Fructose
done
clear
C)
Sucrose
done
clear
D)
Maltose
done
clear
View Answer play_arrow