A) \[{{[Co{{F}_{6}}]}^{3-}}\]
B) \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
C) \[{{[Fe{{(CN)}_{6}}]}^{3-}}\]
D) \[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
Correct Answer: A
Solution :
\[{{[Co{{F}_{6}}]}^{3-}}\]is a outer orbital complex ion. It involves outer orbital hybridisation. It has \[s{{p}^{3}}{{d}^{2}}-\]hybridisation because \[{{F}^{-}}\]is a weak ligand.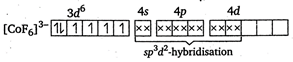
You need to login to perform this action.
You will be redirected in
3 sec
