A) \[Xe{{F}_{2}}\]
B) \[Xe{{O}_{3}}F\]
C) \[Xe{{O}_{2}}{{F}_{2}}\]
D) \[Xe{{F}_{4}}\]
Correct Answer: A
Solution :
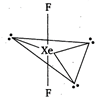 \[\text{Xe}{{\text{F}}_{\text{2}}}\]molecule contains two bond pairs and three lone pairs in the outer-shell of central atom and thus its hybridisation is\[s{{p}^{3}}{{d}^{2}}\] but to minimize the repulsive forces the three lone pairs occupy the equatorial position and the molecule becomes linear shape.
\[\text{Xe}{{\text{F}}_{\text{2}}}\]molecule contains two bond pairs and three lone pairs in the outer-shell of central atom and thus its hybridisation is\[s{{p}^{3}}{{d}^{2}}\] but to minimize the repulsive forces the three lone pairs occupy the equatorial position and the molecule becomes linear shape.
You need to login to perform this action.
You will be redirected in
3 sec
