A)
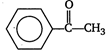
B) \[C{{H}_{3}}OH\]
C) \[C{{H}_{3}}C{{H}_{2}}OH\]
D) \[C{{H}_{3}}-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-C{{H}_{3}}\]
Correct Answer: B
Solution :
The compounds having either \[C{{H}_{3}}-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-\] group or\[C{{H}_{3}}-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-\]group when treated with \[{{\text{I}}_{\text{2}}}\]and \[\text{NaOH,}\]give yellow precipitate of iodoform. \[\therefore \]\[C{{H}_{3}}OH\] does not give iodoform. Note:\[C{{H}_{3}}C{{H}_{2}}OH\]is oxidised to \[C{{H}_{3}}CHO\]and hence, give positive iodoform test.You need to login to perform this action.
You will be redirected in
3 sec
