A) \[IV>II>I>III\]
B) \[II>IV>m>l\]
C) \[II>IV>I>III\]
D) \[IV>II>III>I\text{ }\]
Correct Answer: C
Solution :
In the presence of electron releasing group, (like\[C{{H}_{3}},C{{H}_{3}}O\]), the positive charge is dispersed more. This dispersal of positive charge stabilises the carbocation (carbonium ion). While electron withdrawing substituent (like\[\text{N}{{\text{O}}_{\text{2}}}\]) destabilises the carbonium ion by intensifying the positive charge. Thus, the order of stability of given benzyl carbonium ions is as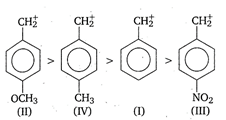
You need to login to perform this action.
You will be redirected in
3 sec
