A) \[C{{H}_{4}}\]
B) \[Xe{{F}_{4}}\]
C) \[{{H}_{2}}O\]
D) \[N{{H}_{3}}\]
Correct Answer: B
Solution :
[a] \[C{{H}_{4}}\to \]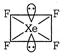 \[=4\sigma \]bond +2 lone pairs \[\Rightarrow \]\[s{{p}^{3}}{{d}^{2}}\]hybridization [c] \[{{H}_{2}}O\to \]
\[=4\sigma \]bond +2 lone pairs \[\Rightarrow \]\[s{{p}^{3}}{{d}^{2}}\]hybridization [c] \[{{H}_{2}}O\to \]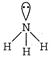 \[=3\sigma \]bond + 1 lone pair \[\Rightarrow \]\[s{{p}^{3}}\]hybridisation
\[=3\sigma \]bond + 1 lone pair \[\Rightarrow \]\[s{{p}^{3}}\]hybridisation
You need to login to perform this action.
You will be redirected in
3 sec
