question_answer 1) Which of the following physical quantity unit is not a fundamental unit?
A)
Length
done
clear
B)
Mass
done
clear
C)
Magnetic field
done
clear
D)
Current
done
clear
View Answer play_arrow
question_answer 2) The dimensional formula of electric potential is
A)
\[M{{L}^{2}}{{T}^{-3}}{{A}^{-1}}\]
done
clear
B)
\[{{M}^{-1}}{{L}^{2}}{{T}^{-2}}A\]
done
clear
C)
\[{{M}^{-1}}{{L}^{2}}{{T}^{-2}}{{A}^{-1}}\]
done
clear
D)
\[M{{L}^{2}}{{T}^{-2}}A\]
done
clear
View Answer play_arrow
question_answer 3) The motion of a particle in straight line is an example of
A)
constant velocity motion
done
clear
B)
uniformly accelerated motion
done
clear
C)
non-uniformly accelerated motion
done
clear
D)
zero velocity motion
done
clear
View Answer play_arrow
question_answer 4) The velocity vector of the motion described by the position vector of a particle,\[r=2ti+{{t}^{2}}\hat{j}\]is given by
A)
\[V=2\hat{i}+2t\,\hat{j}\]
done
clear
B)
\[V=2t\,\hat{i}+2t\,\hat{j}\]
done
clear
C)
\[V=t\,\hat{i}+{{t}^{2}}\,\hat{j}\]
done
clear
D)
\[V=2\,\hat{i}+{{t}^{2}}\,\hat{j}\]
done
clear
View Answer play_arrow
question_answer 5) The velocity-time graph of particle comes out to be a non-linear curve. The motion is
A)
uniform velocity motion
done
clear
B)
uniformly accelerated motion
done
clear
C)
non-uniform accelerated motion
done
clear
D)
nothing can be said about the motion
done
clear
View Answer play_arrow
question_answer 6) A projectile is thrown with initial velocity\[{{\upsilon }_{0}}\]and angle\[30{}^\circ \]with the horizontal. If it remains in the air for 1 s, what was its initial velocity?
A)
19.6m/s
done
clear
B)
9.8 m/s
done
clear
C)
4.9 m/s
done
clear
D)
1 m/s
done
clear
View Answer play_arrow
question_answer 7) Newtons second law of motion is
A)
\[F=dp/dt\]
done
clear
B)
\[F=mv\]
done
clear
C)
\[F=m{{v}^{2}}\]
done
clear
D)
\[F={{m}^{2}}v\]
done
clear
View Answer play_arrow
question_answer 8) The centripetal force is given by the expression
A)
\[\frac{M{{v}^{2}}}{R}\]
done
clear
B)
\[\frac{{{M}^{2}}v}{R}\]
done
clear
C)
\[\frac{Mv}{{{R}^{2}}}\]
done
clear
D)
\[\frac{Mv}{R}\]
done
clear
View Answer play_arrow
question_answer 9) Uniform circular motion is an example of
A)
constant speed motion
done
clear
B)
constant velocity motion
done
clear
C)
non-accelerated motion
done
clear
D)
zero accelerated motion
done
clear
View Answer play_arrow
question_answer 10) The scalar product of two vectors \[A=2\hat{i}+2\hat{j}-\hat{k}\] and\[B=-\text{ }\hat{j}+\hat{k},\]is given by
A)
\[A.B=3\]
done
clear
B)
\[A.B=4\]
done
clear
C)
\[A.B=-4\]
done
clear
D)
\[A.B=-3\]
done
clear
View Answer play_arrow
question_answer 11) The linear momentum is conserved in
A)
elastic collisions
done
clear
B)
inelastic collisions
done
clear
C)
Both 1 and 2
done
clear
D)
Neither 1 nor 2
done
clear
View Answer play_arrow
question_answer 12) The power (P) of an engine lifting a mass of 100 kg upto a height of 10 m in 1 min is
A)
P=163.3W
done
clear
B)
P = 9800 W
done
clear
C)
P = 10000W
done
clear
D)
P = 5000 W
done
clear
View Answer play_arrow
question_answer 13) The conservation of angular momentum demands that
A)
the external force on the system must be zero
done
clear
B)
the external torque on the system must be zero
done
clear
C)
both the external force as well as the external torque must be zero
done
clear
D)
neither of them must be zero
done
clear
View Answer play_arrow
question_answer 14) The moment of inertia (7) and the angular momentum (L) are related by the expression
A)
\[I=L\omega \]
done
clear
B)
\[L=I\omega \]
done
clear
C)
\[L={{I}^{2}}\omega \]
done
clear
D)
\[\omega =LI\] where co is the angular velocity.
done
clear
View Answer play_arrow
question_answer 15) The moment of inertia\[(I)\]of a sphere of radius R and mass M is given by
A)
\[I=M{{R}^{2}}\]
done
clear
B)
\[I=(1/2)M{{R}^{2}}\]
done
clear
C)
\[I=(4/3)M{{R}^{2}}\]
done
clear
D)
\[I=(2/5)M{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 16) The universal law of gravitation is the force law known also as the
A)
triangular law
done
clear
B)
square law
done
clear
C)
inverse square law
done
clear
D)
parallelogram law
done
clear
View Answer play_arrow
question_answer 17) The value of acceleration due to gravity at the surface of earth
A)
is maximum at the poles
done
clear
B)
is maximum at the equator
done
clear
C)
remains constant everywhere on the surface of the earth
done
clear
D)
is maximum at the intenational timeline
done
clear
View Answer play_arrow
question_answer 18) The escape velocity of a particle from the surface of the earth is given by
A)
\[{{(gR)}^{1/2}}\]
done
clear
B)
\[{{(2gR)}^{1/2}}\]
done
clear
C)
\[{{(3gR)}^{1/2}}\]
done
clear
D)
\[{{(gR/2)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 19) The Youngs modulus of a rope of 10 m length and having diameter of 2 cm is\[200\times {{10}^{11}}\]dyne\[/c{{m}^{2}}\]. If the elongation produced in the rope is 1 cm, the force applied on the rope is
A)
\[6.28\times {{10}^{5}}N\]
done
clear
B)
\[6.28\times {{10}^{4}}N\]
done
clear
C)
\[6.28\times {{10}^{4}}\]dyne
done
clear
D)
\[6.28\times {{10}^{5}}\]dyne
done
clear
View Answer play_arrow
question_answer 20) The pressure exerted at any point in an enclosed fluid is transmitted equally in all directions. This is known as
A)
Archimedes principle
done
clear
B)
Law of floatation
done
clear
C)
Pascals law
done
clear
D)
Bernoullis principle
done
clear
View Answer play_arrow
question_answer 21)
There are two identical small holes on the opposite sides of a tank containing a liquid. The tank is open at the top. The difference in height between the two holes is h. As the liquid comes out of the two holes, the tank will experience a net horizontal force proportional to
A)
\[\sqrt{h}\]
done
clear
B)
\[h\]
done
clear
C)
\[{{h}^{3/2}}\]
done
clear
D)
\[{{h}^{2}}\]
done
clear
View Answer play_arrow
question_answer 22) The zeroth law of thermodynamics for three systems A, B and C in contact demands that
A)
A and B are in thermal equilibrium
done
clear
B)
B and C are in thermal equilibrium
done
clear
C)
A and C are in thermal equilibrium
done
clear
D)
A B and C are in thermal equilibrium
done
clear
View Answer play_arrow
question_answer 23) The efficiency of a Carnot engine kept at the temperatures of\[27{}^\circ C\]and\[127{}^\circ C\]is
A)
20%
done
clear
B)
25%
done
clear
C)
30%
done
clear
D)
40%
done
clear
View Answer play_arrow
question_answer 24) The average pressure of an ideal gas is
A)
\[p=(1/3)mnV_{av}^{2}\]
done
clear
B)
\[p=(1/2)mn{{V}_{av}}\]
done
clear
C)
\[p=(1/4)mnV_{av}^{2}\]
done
clear
D)
\[p=(1/3)mn{{V}_{av}}\]
done
clear
View Answer play_arrow
question_answer 25) where symbols have their usual meanings. According to equipartition law of energy each particle in a system of particles have thermal energy E equal to
A)
\[E={{k}_{B}}T\]
done
clear
B)
\[E=(1/2){{k}_{B}}T\]
done
clear
C)
\[E=3{{k}_{B}}T\]
done
clear
D)
\[E=(3/2){{k}_{B}}T\]
done
clear
View Answer play_arrow
question_answer 26) The velocity of sound in a gas is 1300 m/s at STP and specific heat at constant pressure is\[6.84\,cal\,{{K}^{-1}}mo{{l}^{-1}}\]. The rms velocity at STP is (R\[=1.98\text{ }cal\text{ }{{K}^{-1}}mo{{l}^{-1}}\])
A)
1300 m/s
done
clear
B)
2600 m/s
done
clear
C)
1898 m/s
done
clear
D)
650 m/s
done
clear
View Answer play_arrow
question_answer 27) The time period of a simple pendulum of length 9.8 m is
A)
0.159s
done
clear
B)
3.14s
done
clear
C)
6.5s
done
clear
D)
6.28s
done
clear
View Answer play_arrow
question_answer 28) The displacement, velocity and acceleration in a simple harmonic motion are related as the
A)
displacement, velocity and acceleration all act in the same direction
done
clear
B)
displacement and velocity act in the same direction but acceleration in the opposite direction
done
clear
C)
velocity and acceleration are parallel and both are perpendicular to the displacement
done
clear
D)
displacement and acceleration are antiparallel and both perpendicular to the velocity
done
clear
View Answer play_arrow
question_answer 29) The beats are the examples of
A)
simple harmonic motion
done
clear
B)
interference of two or more waves having same amplitude but slightly different frequencies in the same direction
done
clear
C)
interference of two or more waves having different amplitude but same frequencies in the same direction
done
clear
D)
interference of two or more waves having same amplitude out slightly different frequencies in the perpendicular direction
done
clear
View Answer play_arrow
question_answer 30) The fundamental frequency of an open pipe of length 1 m, if the speed of sound in air is 340 m/s is
A)
340 Hz
done
clear
B)
170 Hz
done
clear
C)
680 Hz
done
clear
D)
85 Hz
done
clear
View Answer play_arrow
question_answer 31) A whistle with frequency 1020 Hz is blown at a station. A man travelling in train moving towards the station at 30 m/s hears the sound of the whistle. If the speed of sound is 340 m/s, the apparent frequency heard by him is
A)
1020 Hz
done
clear
B)
1110 Hz
done
clear
C)
2040 Hz
done
clear
D)
610 Hz
done
clear
View Answer play_arrow
question_answer 32) An electric charge does not have which of hte following properties?
A)
Total charge conservation
done
clear
B)
Quantization of charge
done
clear
C)
Two type of charge
done
clear
D)
Circular line of force
done
clear
View Answer play_arrow
question_answer 33) The net electric force on a charge of\[+3\mu C\]at the mid-point on the line joining two charges of magnitude\[+2\mu C\]and\[-2\mu C\]separated by the distance of 6 mm, is
A)
6000 N
done
clear
B)
500 N
done
clear
C)
60 N
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 34) A hollow sphere of radius 0.1 m has a charge of\[5\times {{10}^{-8}}C\]. The potential at a distance of 5 cm from the centre of the sphere is \[\left( \frac{1}{4\pi {{\varepsilon }_{0}}}=9\times {{10}^{9}}N{{m}^{2}}{{C}^{-2}} \right)\]
A)
4000V
done
clear
B)
4500V
done
clear
C)
5000V
done
clear
D)
6000V
done
clear
View Answer play_arrow
question_answer 35) A parallel plate capacitor of capacitance 5 microfarad is charged to 120 V and then connected to another uncharged capacitor. If the potential falls to 40 V, and capacitance of the second capacitor is
A)
5 microfarad
done
clear
B)
10 microfarad
done
clear
C)
15 microfarad
done
clear
D)
20 microfarad
done
clear
View Answer play_arrow
question_answer 36) Two identical capacitors are first connected in series and then in parallel. The ratio of equivalent capacitance is
A)
\[1:1\]
done
clear
B)
\[1:2\]
done
clear
C)
\[1:3\]
done
clear
D)
\[1:4\]
done
clear
View Answer play_arrow
question_answer 37) An electron revolves in a circle at the rate of \[{{10}^{19}}\]rounds per second. The equivalent current is\[(e=1.6\times {{10}^{-19}}C)\]
A)
1.0 A
done
clear
B)
1.6 A
done
clear
C)
2.0 A
done
clear
D)
2.6 A
done
clear
View Answer play_arrow
question_answer 38) A silver wire of radius 0.1 cm carries a current of2A. If the charge density in silver is \[5.86\times {{10}^{28}}{{m}^{-3}},\]the drift velocity is
A)
\[0.2\times {{10}^{-3}}m/s\]
done
clear
B)
\[0.4\times {{10}^{-4}}m/s\]
done
clear
C)
\[0.68\times {{10}^{-4}}m/s\]
done
clear
D)
\[7\times {{10}^{-4}}m/s\]
done
clear
View Answer play_arrow
question_answer 39) Aim long wire of diameter of 0.31 mm has a resistance of\[4.2\,\Omega \]. If it is replaced by another wire of same material of length 1.5 m and diameter 0.155 mm, the resistance of wire is
A)
\[25.2\,\Omega \]
done
clear
B)
\[0.6\,\Omega \]
done
clear
C)
\[26.7\,\Omega \]
done
clear
D)
\[0.8\,\Omega \]
done
clear
View Answer play_arrow
question_answer 40) cells of emf 1.5 V each having internal resistance of 1 ohm are connected to an external resistance of 1.5 ohms. To get maximum current
A)
all cells are connected in series combination
done
clear
B)
all cells are connected in parallel combination
done
clear
C)
4 cells in each row are connected in series and 6 such rows are connected in parallel
done
clear
D)
6 cells in each row are connected in series and 4 such rows are connected in parallel
done
clear
View Answer play_arrow
question_answer 41) The temperature coefficient of the resistance of a wire is 0.00125 per\[{}^\circ C\]. At 300 K its resistance is\[1\,\Omega \]. The resistance of wire will be\[2\,\,\Omega \]at
A)
1154K
done
clear
B)
1100K
done
clear
C)
1400 K
done
clear
D)
1127K
done
clear
View Answer play_arrow
question_answer 42) A long straight wire is carrying a current of 12 A. The magnetic field at a distance of 8 cm is\[({{\mu }_{0}}=4\pi \times {{10}^{-7}}N/{{A}^{2}})\]
A)
\[2\times {{10}^{-4}}Wb/{{m}^{2}}\]
done
clear
B)
\[3\times {{10}^{-5}}Wb/{{m}^{2}}\]
done
clear
C)
\[4\times {{10}^{-4}}Wb/{{m}^{2}}\]
done
clear
D)
\[4\times {{10}^{-5}}Wb/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 43) The magnetic field at a point on the axis of a long solenoid having 5 turns per cm length when a current of 0.8 A flows through it is
A)
\[5.024\times {{10}^{-8}}Wb/{{m}^{2}}\]
done
clear
B)
\[6.024\times {{10}^{-8}}Wb/{{m}^{2}}\]
done
clear
C)
\[7.024\times {{10}^{-8}}Wb/{{m}^{2}}\]
done
clear
D)
\[8.024\times {{10}^{-8}}Wb/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 44) Two straight wires each 10 cm long are parallel to one another and separated by 2 cm. When the current flowing in them is 30 A and 40 A respectively, the force experienced by either of the wires is
A)
\[1.2\times {{10}^{-3}}N\]
done
clear
B)
\[12\times {{10}^{-3}}N\]
done
clear
C)
\[11.2\times {{10}^{-3}}N\]
done
clear
D)
\[10.2\times {{10}^{-3}}N\]
done
clear
View Answer play_arrow
question_answer 45) The horizontal and vertical components of earths magnetic field at a place are 0.3 G and 0.52 G. The earths magnetic field and the angle of dip are
A)
\[0.3\text{ }G\text{ }and\text{ }\delta =30{}^\circ \]
done
clear
B)
\[0.4\text{ }G\text{ }and\text{ }\delta =40{}^\circ \]
done
clear
C)
\[0.5\text{ }G\text{ }and\text{ }\delta =50{}^\circ \]
done
clear
D)
\[0.6\text{ }G\text{ }and\text{ }\delta =60{}^\circ \]
done
clear
View Answer play_arrow
question_answer 46) A bar magnet of pole strength 10 Am is cut into two equal parts breadthwise. The pole strength of each magnet is
A)
5 Am
done
clear
B)
10 Am
done
clear
C)
15 Am
done
clear
D)
20 Am
done
clear
View Answer play_arrow
question_answer 47) A conductor of length 5 cm is moved parallel to itself with a speed of 2 m/s, perpendicular to a uniform magnetic field of\[{{10}^{-3}}Wb/{{m}^{3}}\]. The induced e.m.f. generated is
A)
\[2\times {{10}^{-3}}V\]
done
clear
B)
\[1\times {{10}^{-3}}V\]
done
clear
C)
\[1\times {{10}^{-4}}V\]
done
clear
D)
\[2\times {{10}^{-4}}V\]
done
clear
View Answer play_arrow
question_answer 48) The induced emf in a coil of 10 H inductance in which current varies from 9 A to 4 A in 0.2 s is
A)
200V
done
clear
B)
250V
done
clear
C)
300V
done
clear
D)
350V
done
clear
View Answer play_arrow
question_answer 49) The alternating current in a circuit is given by\[I=50\,sin\,314t\]. The peak value and frequency of the current are
A)
\[{{I}_{0}}=25\,A\]and\[r=100\,Hz\]
done
clear
B)
\[{{I}_{0}}=50\,A\]and\[r=50\,Hz\]
done
clear
C)
\[{{I}_{0}}=50\,A\]and\[r=100\,Hz\]
done
clear
D)
\[{{I}_{0}}=25\,A\]and\[r=50\,Hz\]
done
clear
View Answer play_arrow
question_answer 50) A 50 Hz AC signal is applied in a circuit of inductance of\[(1/\pi )H\]and resistance\[2100\text{ }\Omega \]. The impedance offered by the circuit is
A)
\[1500\text{ }\Omega \]
done
clear
B)
\[1700\text{ }\Omega \]
done
clear
C)
\[2102\text{ }\Omega \]
done
clear
D)
\[2500\text{ }\Omega \]
done
clear
View Answer play_arrow
question_answer 51) If the alternating current\[I={{I}_{1}}\cos \omega t+{{I}_{2}}\] \[\sin \omega t\] then the rms current is given by
A)
\[\frac{{{I}_{1}}+{{I}_{2}}}{\sqrt{2}}\]
done
clear
B)
\[\frac{|{{I}_{1}}+{{I}_{2}}|}{\sqrt{2}}\]
done
clear
C)
\[\sqrt{\left( \frac{I_{1}^{2}+I_{2}^{2}}{\sqrt{2}} \right)}\]
done
clear
D)
\[\sqrt{\frac{I_{1}^{2}+I_{2}^{2}}{\sqrt{2}}}\]
done
clear
View Answer play_arrow
question_answer 52) The transverse nature of electromagnetic waves is proved by which of the following?
A)
Interference phenomena
done
clear
B)
Diffraction phenomena
done
clear
C)
Dispersion phenomena
done
clear
D)
Polarization phenomena
done
clear
View Answer play_arrow
question_answer 53) Which component of electromagnetic spectrum have maximum wavelength?
A)
Radio waves
done
clear
B)
Visible spectrum
done
clear
C)
Gamma rays
done
clear
D)
X-rays
done
clear
View Answer play_arrow
question_answer 54) An object is 8 cm high. It is desired to form a real image 4 cm high at 60 cm from the mirror. The type of mirror needed with the focal length is
A)
convex mirror with focal length\[f=40\text{ }cm\]
done
clear
B)
convex mirror with focal length \[f=20\text{ }cm\]
done
clear
C)
concave mirror with focal length\[f=-40\text{ }cm\]
done
clear
D)
concave mirror with focal length\[f=-20\text{ }cm\]
done
clear
View Answer play_arrow
question_answer 55) When an object is placed 40 cm from a diverging lens, its virtual image is formed 20 cm from the lens. The focal length and power of lens are
A)
\[F=-20cm,P=-5D\]
done
clear
B)
\[F=-40cm,P=-5D\]
done
clear
C)
\[F=-40cm,P=-2.5D\]
done
clear
D)
\[F=-20cm,\text{ }P=-2.5\text{ }D\]
done
clear
View Answer play_arrow
question_answer 56) A magnifying glass of focal length 5 cm is used to view an object by a person whose smallest distance of distinct vision is 25 cm. If he holds the glass close to eye, the magnification is
A)
5
done
clear
B)
6
done
clear
C)
2.5
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 57) A person has a minimum distance of distinct vision as 50 cm. The power of lenses required to read a book at a distance of 25 cm is
A)
3D
done
clear
B)
1D
done
clear
C)
2D
done
clear
D)
4D
done
clear
View Answer play_arrow
question_answer 58) If two slits in Youngs experiment are 0.4 mm apart and fringe width on a screen 200 cm away is 2 mm the wavelength of light illuminating the slits is
A)
500 nm
done
clear
B)
600 nm
done
clear
C)
400 nm
done
clear
D)
300 nm
done
clear
View Answer play_arrow
question_answer 59) Electric field strength due to a diple at a point on the axial line of dipole is
A)
from positive charge to negative charge
done
clear
B)
from negative charge to positive charge
done
clear
C)
along the equatorial line
done
clear
D)
at an angle to axial line
done
clear
View Answer play_arrow
question_answer 60) The distance of moon form the earth is \[3.8\times {{10}^{5}}\]km. Supposing that the eye is most sensitive to the light of wavelength 550 nm, the separation of two points on the moon that can be resolved by a 500 cm telescope is
A)
50 m
done
clear
B)
55 m
done
clear
C)
51 m
done
clear
D)
60 m
done
clear
View Answer play_arrow
question_answer 61) Unpolarized light falls on two polarizing sheets placed one on top of other. If the intensity of transmitted light is one fourth of the incident light, the angle between them is
A)
\[35{}^\circ \]
done
clear
B)
\[40{}^\circ \]
done
clear
C)
\[45{}^\circ \]
done
clear
D)
\[50{}^\circ \]
done
clear
E)
None of the above
done
clear
View Answer play_arrow
question_answer 62) The Brewsters law is given by the expression
A)
\[\mu =\frac{\sin i}{\sin r}\]
done
clear
B)
\[\mu =\tan {{\theta }^{p}}\]
done
clear
C)
\[\mu =\cos \theta \]
done
clear
D)
\[\mu =\sin \theta \]
done
clear
View Answer play_arrow
question_answer 63) Einstein photoelectric equation is
A)
\[{{E}_{\max }}=hv-\phi \]
done
clear
B)
\[E=m{{c}^{2}}\]
done
clear
C)
\[{{E}^{2}}={{p}^{2}}{{c}^{2}}+m_{0}^{2}{{c}^{4}}\]
done
clear
D)
\[E=\left( \frac{1}{2} \right)m{{v}^{2}}\]
done
clear
View Answer play_arrow
question_answer 64) The Davission-Germer experiment is the direct evidence of
A)
particle nature of electrons
done
clear
B)
wave nature of electrons
done
clear
C)
wave nature of light
done
clear
D)
particle nature of light
done
clear
View Answer play_arrow
question_answer 65) The Rutherford scattering experiment porves that an atom consists of
A)
a sphere of positive charge in which electrons are embedded like seeds of water-melon
done
clear
B)
a sphere of negative charge in which protons are embedded like seeds of water-melon
done
clear
C)
a sphere of electron cloud in which the positive charge in placed at the centre of the sphere
done
clear
D)
a sphere of neutral charge
done
clear
View Answer play_arrow
question_answer 66) According to Bohr model of hydrogen atom, only those orbits are permissible which satisfy the condition
A)
\[mv=nh\]
done
clear
B)
\[\frac{m{{v}^{2}}}{r}=n\left( \frac{h}{2\pi } \right)\]
done
clear
C)
\[mvr=n\left( \frac{h}{2\pi } \right)\]
done
clear
D)
\[mv{{r}^{2}}=n\left( \frac{h}{2\pi } \right)\]
done
clear
View Answer play_arrow
question_answer 67) The radioactive decay of thorium (A = 232, Z = 90) releases six alpha and four beta particles. The atomic number and mass number of the final product is
A)
Z = 80, A = 207
done
clear
B)
Z = 82, A = 208
done
clear
C)
Z = 92, A = 209
done
clear
D)
Z=90, A=207
done
clear
View Answer play_arrow
question_answer 68) Polonium has a half-life of 140 days. If we take 20 g of polonium initially then the amount of it that remains after 280 days is
A)
2.5 g
done
clear
B)
5 g
done
clear
C)
10 g
done
clear
D)
15 g
done
clear
View Answer play_arrow
question_answer 69) Based on the band theory of conductors, insulators and semi-conductors, the forbidden gap is smallest in
A)
conductors
done
clear
B)
insulators
done
clear
C)
semi-conductors
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 70) Based on the I-V characteristics of the diode, we can classify diode as
A)
bi-directional device
done
clear
B)
ohmic device
done
clear
C)
non-ohmic device
done
clear
D)
passive element
done
clear
View Answer play_arrow
question_answer 71) The Boolean expression for the XOR gate is
A)
\[Y=A+B\]
done
clear
B)
\[Y=A-B\]
done
clear
C)
\[V=AB-BA\]
done
clear
D)
\[V=A\oplus B\]
done
clear
View Answer play_arrow
question_answer 72) Which of the following logic gates are also known as the Universal gates?
A)
AND, OR, NOT gate
done
clear
B)
XOR, XNOR gate
done
clear
C)
NAND, NOR gate
done
clear
D)
All logic gates
done
clear
View Answer play_arrow
question_answer 73) The length of antenna (L) required to propagate a signal of wavelength\[\lambda \]is given as
A)
\[L=\frac{\lambda }{2}\]
done
clear
B)
\[L=2\lambda \]
done
clear
C)
\[L=\frac{\lambda }{3}\]
done
clear
D)
\[L=\frac{\lambda }{4}\]
done
clear
View Answer play_arrow
question_answer 74) The modulation is the process in which the
A)
modulating signal is sent by antenna in the air
done
clear
B)
carrier signal is sent by the antenna in the air
done
clear
C)
modulated signal formed by the mixing of modulating signal with the carrier signal is sent by the antenna
done
clear
D)
modulated signal formed by the mixing of modulating signal with the carrier signal is received by a receiver antenna
done
clear
View Answer play_arrow
question_answer 75) The demodulator or detector circuit consists of a
A)
resistor
done
clear
B)
transistor
done
clear
C)
diode
done
clear
D)
capacitor
done
clear
View Answer play_arrow
question_answer 76) Which of the following would contain the same number of atoms as 20 g of calcium? [At. masses:\[Ca=40,\text{ }Mg=24,\text{ }C=12\]]
A)
24 g of magnesium
done
clear
B)
12 g of carbon
done
clear
C)
24 g of carbon
done
clear
D)
12 g of magnesium
done
clear
View Answer play_arrow
question_answer 77) 100 mL of\[1N\,{{H}_{2}}S{{O}_{4}}\]is mixed with 100 mL of 1 \[M\text{ }NaOH\]solution. The resulting Solution will be
A)
highly acidic
done
clear
B)
neutral
done
clear
C)
highly basic
done
clear
D)
slightly acidic
done
clear
View Answer play_arrow
question_answer 78) The bond order of N3 on the basis of molecular orbital theory is [Atomic number of\[N=71\]
A)
3
done
clear
B)
2.5
done
clear
C)
2
done
clear
D)
1.5
done
clear
View Answer play_arrow
question_answer 79) What is the total number of electrons that can have the values\[n=2,\text{ l}=1,\text{ }s=1/2\]in the electronic configuration\[1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}\]?
A)
1
done
clear
B)
3
done
clear
C)
5
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 80) Calculate the wavelength associated with an electron moving with a velocity of\[106\text{ }m/s\](mass of electron\[=9.1\times {{10}^{-31}}kg,\]\[h=6.6\times {{10}^{-34}}kg\,{{m}^{2}}{{s}^{-1}}\])
A)
\[6.2\times {{10}^{-8}}m\]
done
clear
B)
\[7.25\times {{10}^{-8}}m\]
done
clear
C)
\[6.25\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 81) Which of the following pairs is not correctly matched?
A)
Hunds rule - In orbitals of equivalent energy electron spins remain unpaired if possible
done
clear
B)
Paulis exclusion principle - No two electrons can have all the four quantum numbers identical
done
clear
C)
Zeeman effect - The effect of magnetic field on the atomatic spectra
done
clear
D)
Uncertainty principle - It is impossible to determine the position of an electron
done
clear
View Answer play_arrow
question_answer 82) The orbital diagram in which Aufbau principle is violated is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 83) The\[{{[OH]}^{-}}\]in a solution is\[1\text{ }mol\text{ }{{L}^{-1}}\]. The pH of the solution is
A)
1
done
clear
B)
0
done
clear
C)
14
done
clear
D)
\[{{10}^{-14}}\]
done
clear
View Answer play_arrow
question_answer 84) The solubility of\[Fe{{(OH)}^{3}}\]is\[x\text{ }mol\text{ }{{L}^{-1}}\]. Its\[{{K}_{sp}}\]would be
A)
\[9{{x}^{3}}\]
done
clear
B)
\[3{{x}^{4}}\]
done
clear
C)
\[27{{x}^{4}}\]
done
clear
D)
\[9{{x}^{4}}\]
done
clear
View Answer play_arrow
question_answer 85) In which of the following reactions, increase in pressure will favour the forward reaction?
A)
\[PC{{l}_{5}}(g)PC{{l}_{3}}(g)+C{{l}_{2}}(g)\]
done
clear
B)
\[2NO(g)+{{O}_{2}}(g)2N{{O}_{2}}(g)\]
done
clear
C)
\[C(s)+{{H}_{2}}O(g)CO(g)+{{H}_{2}}(g)\]
done
clear
D)
\[2HI(g){{H}_{2}}(g)+{{I}_{2}}(g)\]
done
clear
View Answer play_arrow
question_answer 86) Which of the following is a Lewis acid?
A)
\[BF_{4}^{-}\]
done
clear
B)
\[O{{H}^{-}}\]
done
clear
C)
\[AlC{{l}_{3}}\]
done
clear
D)
\[RN{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 87) According to collision theory of chemical reactions rates of the reaction increase with increase in the temperature of a reaction because of
A)
increase in the velocity of the reacting molecules
done
clear
B)
increase in the number of collisions
done
clear
C)
increase in the number of molecules having the activation energy (threshold energy)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 88) Consider the reaction, \[2{{N}_{2}}{{O}_{5}}(g)\xrightarrow[{}]{{}}4N{{O}_{2}}(g)+{{O}_{2}}(g)\] The rate law for this reaction is \[rate=k[{{N}_{2}}{{O}_{5}}]\] Which of the following statements is true regarding the above reaction?
A)
Its order is 1 and molecularity is 1
done
clear
B)
Its order is 1 and molecularity is 2
done
clear
C)
Its order is 2 and molecularity is 2
done
clear
D)
Its order is 2 and molecularity is 1
done
clear
View Answer play_arrow
question_answer 89) A catalyst is a substance which
A)
increases the rate of forward reaction in reversible reaction
done
clear
B)
increases the rate of both forward and backward reaction in a reversible reaction
done
clear
C)
does not influence a reversible reaction
done
clear
D)
increases the rate of backward reaction in a reversible reaction.
done
clear
View Answer play_arrow
question_answer 90) Which of the following solutions will have the highest boiling point?
A)
1 M glucose solution
done
clear
B)
1 M sodium nitrate solution
done
clear
C)
1 M barium chloride solution
done
clear
D)
1 M aluminium chloride solution
done
clear
View Answer play_arrow
question_answer 91) Which of the following is a colligative property?
A)
Lowering of vapour pressure
done
clear
B)
Osmotic pressure
done
clear
C)
Boiling point
done
clear
D)
Change in entropy
done
clear
View Answer play_arrow
question_answer 92) vant Hoff factor for\[Ca{{(N{{O}_{3}})}_{2}}\]is
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 93) What will be the freezing point of 1% solution of glucose in water, given that molal depression constant for water is\[1.84\text{ }K\text{ }mo{{l}^{-1}}\]
A)
272.898 K
done
clear
B)
\[0.102{}^\circ C\]
done
clear
C)
273 K
done
clear
D)
\[0.108{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 94) If\[\Delta H\]and\[\Delta S\]are positive for a reaction, the reaction will be spontaneous only when
A)
\[T\Delta S=\Delta H\]
done
clear
B)
\[T\Delta S>\Delta H\]
done
clear
C)
\[T\Delta S<\Delta H\]
done
clear
D)
\[T\Delta S\]is negative
done
clear
View Answer play_arrow
question_answer 95) Calculate the enthalpy change for the reaction, \[{{C}_{2}}{{H}_{4}}(g)+{{H}_{2}}(g)\xrightarrow[{}]{{}}{{C}_{2}}{{H}_{6}}(g)\] using the data given below \[{{C}_{2}}{{H}_{4}}(g)+3{{O}_{2}}(g)\xrightarrow[{}]{{}}2C{{O}_{2}}(g)+2{{H}_{2}}O(l)\] \[\Delta H=-\text{ }1415\text{ }kJ\] \[{{C}_{2}}{{H}_{6}}(g)+\frac{7}{2}{{O}_{2}}(g)\xrightarrow[{}]{{}}2C{{O}_{2}}(g)+3{{H}_{2}}O\] \[\Delta H=-1566\text{ }kJ\] \[{{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow[{}]{{}}{{H}_{2}}O(l);\] \[\Delta H=-286\text{ }kJ\]
A)
\[-437kJ\]
done
clear
B)
135kJ
done
clear
C)
\[-135\text{ }kJ\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 96) In thermodynamics, a quantity whose value simply depends upon the initial and final state of the system is called
A)
a thermodynamic quantity
done
clear
B)
a state function
done
clear
C)
an adiabatic quantity
done
clear
D)
a path function
done
clear
View Answer play_arrow
question_answer 97) All naturally occurring processes proceed spontaneously in a direction which leads to
A)
increase in enthalpy of system
done
clear
B)
decrease in entropy of system
done
clear
C)
decrease in free energy of system
done
clear
D)
increase in free energy of system
done
clear
View Answer play_arrow
question_answer 98) When an electrolytic solution conducts electricity, the current is carried by
A)
the electrons
done
clear
B)
cations and anions
done
clear
C)
neutral molecules
done
clear
D)
the atoms of the electrolyte
done
clear
View Answer play_arrow
question_answer 99) An electrochemical cell has two half cell reactions as, \[{{A}^{2+}}+2{{e}^{-}}\xrightarrow[{}]{{}}A;\] \[E_{{{A}^{2+}}/A}^{0}=0.34\,V\] \[X\xrightarrow[{}]{{}}{{X}^{2+}}+2{{e}^{-}};\]\[E_{{{X}^{2+}}/X}^{0}=-2.37\,V\] The cell voltage will be
A)
2.71 V
done
clear
B)
2.03 V
done
clear
C)
\[-2.71\text{ }V\]
done
clear
D)
\[-2.03V\]
done
clear
View Answer play_arrow
question_answer 100) In the electrolysis of dilute\[{{H}_{2}}S{{O}_{4}}\]using platinum electrode
A)
\[{{H}_{2}}\] is liberated at cathode
done
clear
B)
\[{{O}_{2}}\] is produced at cathode
done
clear
C)
\[C{{l}_{2}}\] is obtained at cathode
done
clear
D)
\[N{{H}_{3}}\] is produced at anode
done
clear
View Answer play_arrow
question_answer 101) When a solution of sodium hydroxide is added to acetic acid solution, the conductivity of the resulting solution will
A)
increase
done
clear
B)
remain unchanged
done
clear
C)
decrease
done
clear
D)
become zero
done
clear
View Answer play_arrow
question_answer 102) The behaviour of a real gas approaches ideal behaviour at
A)
low temperature, low pressure
done
clear
B)
high temperature, high pressure
done
clear
C)
low temperature, high pressure
done
clear
D)
high temperature, low pressure
done
clear
View Answer play_arrow
question_answer 103) Which of the following is not the postulate of the kinetic theory of gases?
A)
Gas molecules are in a permanent state of random motion
done
clear
B)
Pressure of gas is due to molecular impacts on the walls
done
clear
C)
The molecules are perfectly elastic
done
clear
D)
The molecular collisions are elastic
done
clear
View Answer play_arrow
question_answer 104) When a cation leaves its normal position in the crystal and moves to some interstitial space, the defect in the crystal is known as
A)
Schottky defect
done
clear
B)
F-centre
done
clear
C)
Frenkei defect
done
clear
D)
non-stoichiometric defect
done
clear
View Answer play_arrow
question_answer 105) Fog is a colloidal system of
A)
gas in liquid
done
clear
B)
liquid in gas
done
clear
C)
gas in gas
done
clear
D)
gas in solid
done
clear
View Answer play_arrow
question_answer 106) The purification of a colloidal solution could be done by
A)
sedimentation
done
clear
B)
ultrafiltration
done
clear
C)
filtration
done
clear
D)
precipitation
done
clear
View Answer play_arrow
question_answer 107) Bakelite is a product of the reaction between
A)
formaldehyde and\[NaOH\]
done
clear
B)
aniline and urea
done
clear
C)
phenol and methanol
done
clear
D)
phenol and chloroform
done
clear
View Answer play_arrow
question_answer 108) How does electron affinity change when we move from left to right in a period in the Periodic Table?
A)
It increases
done
clear
B)
It decreases
done
clear
C)
It remains unchanged
done
clear
D)
It first increases and then decreases
done
clear
View Answer play_arrow
question_answer 109) Which of the following statements is not correct?
A)
lonisation energy increases on going down a group in the Periodic Table
done
clear
B)
Among alkaline earth metals, reducing character increases down the group
done
clear
C)
Fluorine is the most electronegative element
done
clear
D)
Metallic character increases on going down a group in the Periodic Table
done
clear
View Answer play_arrow
question_answer 110) Which of the following species has a trigonal planar shape?
A)
\[{}_{\bullet }^{\bullet }CH_{3}^{-}\]
done
clear
B)
\[CH_{3}^{+}\]
done
clear
C)
\[BF_{4}^{-}\]
done
clear
D)
\[Si{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 111) Which of the following will have maximum dipole moment?
A)
\[N{{F}_{3}}\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[C{{H}_{4}}\]
done
clear
D)
\[PC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 112) Which of the following forces is the strongest?
A)
Hydrogen bonding
done
clear
B)
Dipole-dipole forces
done
clear
C)
van der Waals forces
done
clear
D)
Coordinate bonding
done
clear
View Answer play_arrow
question_answer 113) Which of the following statements is correct?
A)
\[s{{p}^{3}}-\]hybrid orbitals have equal s and? character
done
clear
B)
The bond angle decreases with the decrease of s character of a hybridized orbital
done
clear
C)
Resonance decreases the stability of a molecule
done
clear
D)
Resonance is due to delocalization of sigma electrons
done
clear
View Answer play_arrow
question_answer 114) Which of the following is the correct order of increasing oxidising character of oxoacids of chlorine?
A)
\[HCl{{O}_{3}}<HCl{{O}_{4}}<HCl{{O}_{2}}<HClO\]
done
clear
B)
\[HCl{{O}_{4}}<HCl{{O}_{3}}<HCl{{O}_{2}}<HClO\]
done
clear
C)
\[HClO<HCl{{O}_{4}}<HCl{{O}_{3}}<HCl{{O}_{2}}\]
done
clear
D)
\[HClO<HCl{{O}_{2}}<HCl{{O}_{3}}<HCl{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 115) Which of the following oxides of group 16 has the highest boiling point?
A)
\[{{H}_{2}}O\]
done
clear
B)
\[{{H}_{2}}S\]
done
clear
C)
\[{{H}_{2}}Se\]
done
clear
D)
\[{{H}_{2}}Te\]
done
clear
View Answer play_arrow
question_answer 116) The +1 oxidation state of thallium is more stable than its +3 oxidation state because of
A)
its atomic size
done
clear
B)
its ionization potential
done
clear
C)
inert pair effect
done
clear
D)
diagonal relationship
done
clear
View Answer play_arrow
question_answer 117) Which of the following statements is false regarding alkali metals?
A)
Alkali metals are soft and can be cut with the help of knife
done
clear
B)
Alkali metals do not occur in free state in nature
done
clear
C)
Alkali metals are highly electropositive elements
done
clear
D)
Alkali metal hydrides are covalent in character
done
clear
View Answer play_arrow
question_answer 118) Among the following outermost configurations of transition metals, which shows the highest oxidation state?
A)
\[3{{d}^{3}}4{{s}^{2}}\]
done
clear
B)
\[3{{d}^{5}}4{{s}^{1}}\]
done
clear
C)
\[3{{d}^{5}}4{{s}^{2}}\]
done
clear
D)
\[3{{d}^{2}}4{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 119) The tendency of transition metals to form stable complexes is due to their
A)
low ionization energies
done
clear
B)
variable oxidation states
done
clear
C)
strong electropositive nature
done
clear
D)
high charge/size ratio and vacant d orbitals
done
clear
View Answer play_arrow
question_answer 120) The transition metal ions are generally paramagnetic in nature because
A)
they have coloured salts
done
clear
B)
they have one or more unpaired\[d-\]electrons
done
clear
C)
they have one or more paired s-electrons
done
clear
D)
they are reducing agents
done
clear
View Answer play_arrow
question_answer 121) The most common oxidation state of lanthanides is
A)
+4
done
clear
B)
+3
done
clear
C)
+6
done
clear
D)
+ 2
done
clear
View Answer play_arrow
question_answer 122) Specify the coordination number of cobalt in\[{{[Co(CN)({{H}_{2}}O){{(en)}^{2}}]}^{2+}}\]
A)
6
done
clear
B)
4
done
clear
C)
0
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 123) Which of the following complexes is square planar and diamagnetic?
A)
\[{{[NiC{{l}_{4}}]}^{2-}}\]
done
clear
B)
\[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
done
clear
C)
\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
D)
\[{{[CuC{{l}_{4}}]}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 124) Which type of isomerism is exhibited by\[[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]?
A)
Coordination isomerism
done
clear
B)
Linkage isomerism
done
clear
C)
Optical isomerism
done
clear
D)
Geometrical isomerism
done
clear
View Answer play_arrow
question_answer 125) Ethylene diamine tetraacetate ion is a
A)
unidentate ligand
done
clear
B)
bidentate ligand
done
clear
C)
pentadentate ligand
done
clear
D)
hexadentate ligand
done
clear
View Answer play_arrow
question_answer 126) Which of the following is an ore of zinc?
A)
Galena
done
clear
B)
Pyrolusite
done
clear
C)
Sphalerite
done
clear
D)
Magnetite
done
clear
View Answer play_arrow
question_answer 127) The impurities associated with the ore after mining are collectively called
A)
flux
done
clear
B)
slag
done
clear
C)
minerals
done
clear
D)
gangue
done
clear
View Answer play_arrow
question_answer 128) During extraction of iron, the iron obtained at the bottom of blast furnace is known as
A)
steel
done
clear
B)
wrought iron
done
clear
C)
cast iron
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 129) Select the molecule which has only one\[\pi -\]bond
A)
\[CH\equiv CH\]
done
clear
B)
\[C{{H}_{2}}=CH-CHO\]
done
clear
C)
\[C{{H}_{3}}CH=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}CH=CHCOOH\]
done
clear
View Answer play_arrow
question_answer 130) Which of the following groups is ortho and para directing?
A)
\[COCl\]
done
clear
B)
\[CHO\]
done
clear
C)
\[OH\]
done
clear
D)
\[COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 131) Amongst the given cations, the most stable carbonium ion is
A)
\[^{+}C{{H}_{3}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}{{C}^{+}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}\overset{+}{\mathop{C}}\,H\]
done
clear
View Answer play_arrow
question_answer 132) The hybridization of carbon atoms in\[C-C\]single bond of\[HC\equiv CCH=C{{H}_{2}}\]is
A)
\[s{{p}^{3}}-s{{p}^{2}}\]
done
clear
B)
\[s{{p}^{2}}-s{{p}^{3}}\]
done
clear
C)
\[sp-s{{p}^{2}}\]
done
clear
D)
\[s{{p}^{3}}-s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 133) The number of optical isomers of the compound\[C{{H}_{3}}CHBrCHBrCOOH\]is
A)
0
done
clear
B)
1
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 134) Electrolysis of an aqueous solution of sodium ethanoate gives
A)
methane
done
clear
B)
ethane
done
clear
C)
butane
done
clear
D)
methyl ethanoate
done
clear
View Answer play_arrow
question_answer 135) Which of the following compounds will exhibit cis-trans (geometrical) isomerism?
A)
2-butene
done
clear
B)
2-butyne
done
clear
C)
2-butanol
done
clear
D)
1-butanol
done
clear
View Answer play_arrow
question_answer 136) The reaction given below is an example of which of the following? \[2C{{H}_{3}}Br+2Na\xrightarrow[{}]{dry\,ether}{{C}_{2}}{{H}_{6}}+2NaBr\]
A)
Reimer-Tiemann reaction
done
clear
B)
Wurtz reaction
done
clear
C)
Hoffman bromamide reaction
done
clear
D)
Aldol condensation
done
clear
View Answer play_arrow
question_answer 137) Chlorobenzene can be prepared by reacting aniline with
A)
hydrochloric acid in the presence of nitrous acid
done
clear
B)
cuprous chloride in the presence of aluminium chloride
done
clear
C)
chlorine in the presence of aluminium chloride
done
clear
D)
nitrous acid followed by heating with cuprous chloride
done
clear
View Answer play_arrow
question_answer 138) Phenol on treatment with cone.\[HN{{O}_{3}}\]gives
A)
o-nitrophenol
done
clear
B)
p-nitrophenol
done
clear
C)
o-and p-nitrophenol
done
clear
D)
2,4,6-trinitrophenol
done
clear
View Answer play_arrow
question_answer 139) Which of the following compounds will be formed when methoxy benzene is reacted with\[HBr\]?
A)
Phenol and bromomethane
done
clear
B)
Methanol and bromobenzene
done
clear
C)
Phenol and methanol
done
clear
D)
Bromobenzene and bromomethane
done
clear
View Answer play_arrow
question_answer 140) When ethanol and \[{{I}_{2}}\]are heated in the presence of\[N{{a}_{2}}C{{O}_{3}},\]the yellow crystals obtained are of
A)
\[{{C}_{2}}{{H}_{5}}I\]
done
clear
B)
\[C{{H}_{3}}I\]
done
clear
C)
\[CH{{I}_{3}}\]
done
clear
D)
\[C{{H}_{2}}{{I}_{2}}\]
done
clear
View Answer play_arrow
question_answer 141) Identify B in the following series of reaction \[C{{H}_{3}}CHO\xrightarrow[{}]{C{{H}_{3}}MgX}A\xrightarrow[{}]{HOH}B\]
A)
2-propanol
done
clear
B)
1-propanol
done
clear
C)
ethanol
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 142) Which of the following compounds has maximum acidic character?
A)
Dichloroacetic acid
done
clear
B)
Acetic acid
done
clear
C)
Trichloroacetic acid
done
clear
D)
Triflouoroacetic acid
done
clear
View Answer play_arrow
question_answer 143) Among the following, the least reactive aldehyde is
A)
\[HCHO\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CHO\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}CHO\]
done
clear
View Answer play_arrow
question_answer 144) Propanal on reaction with lithium aluminium hydride gives
A)
1-propanol
done
clear
B)
2-propanol
done
clear
C)
ethanol
done
clear
D)
butanol
done
clear
View Answer play_arrow
question_answer 145) Idenitfy Z in the following sequence \[C{{H}_{3}}C{{H}_{2}}I\xrightarrow[{}]{KCN}X\xrightarrow[{}]{{{H}_{3}}{{O}^{+}}/{{H}_{2}}O}Y\] \[\xrightarrow[\Delta ]{{{H}_{3}}{{O}^{+}}/{{H}_{2}}O}Z\]
A)
\[C{{H}_{3}}COCl\]
done
clear
B)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}COOH\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 146) Treatment of aniline with bromine water produces
A)
2,4,6-tribromoaniline
done
clear
B)
a mixture of ortho and para bromoaniline
done
clear
C)
bromobenzene
done
clear
D)
N-bromoaniline
done
clear
View Answer play_arrow
question_answer 147) \[{{C}_{6}}{{H}_{5}}COCl\xrightarrow[{}]{N{{H}_{3}}}X\xrightarrow[{}]{{{P}_{2}}{{O}_{5}}}Y\xrightarrow[{{H}_{2}}]{Ni}Z\]The end product in the above sequence of reactions is
A)
benzoic acid
done
clear
B)
aniline
done
clear
C)
benzyl amine
done
clear
D)
benzonitrile
done
clear
View Answer play_arrow
question_answer 148) Which of the following is least reactive to nitration?
A)
Benzene
done
clear
B)
Nitrobenzene
done
clear
C)
Chlorobenzene
done
clear
D)
Aniline
done
clear
View Answer play_arrow
question_answer 149) Nucleic acids are polymers of
A)
nucleotides
done
clear
B)
nucleosides
done
clear
C)
nuclei of heavy metals
done
clear
D)
proteins
done
clear
View Answer play_arrow
question_answer 150) Which of the following enzymes helps in digestion of proteins?
A)
Invertase
done
clear
B)
Trypsin
done
clear
C)
Tryosinase
done
clear
D)
Urease
done
clear
View Answer play_arrow
question_answer 151) Unicellular algae, diatoms and protozoans are the members of
A)
Monera
done
clear
B)
Fungi
done
clear
C)
Protista
done
clear
D)
Plantae
done
clear
View Answer play_arrow
question_answer 152) Which of the following is also known as amphibian of the plant kingdom?
A)
Algae
done
clear
B)
Bryophytes
done
clear
C)
Pteridophytes
done
clear
D)
Gymnosperms
done
clear
View Answer play_arrow
question_answer 153) Yeast is not included in protozoas in fungi because
A)
chlorophyll is absent
done
clear
B)
it has eukaryotic organisation
done
clear
C)
cell wall is made up of cellulose and reserve food material as starch
done
clear
D)
some fungal hyphae grow in such a way that they give the appearance of pseudomycelium
done
clear
View Answer play_arrow
question_answer 154) An association between roots of higher plants and fungi is called
A)
lichen
done
clear
B)
fern
done
clear
C)
mycorrhiza
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 155) The arrangement of vascular bundles in dicot roots are
A)
conjoint
done
clear
B)
collateral
done
clear
C)
radial
done
clear
D)
bicollateral
done
clear
View Answer play_arrow
question_answer 156) An inflorescence with a single central achlamydeous female flower surrounded by a member of achlamydeous male flower is
A)
cyathium
done
clear
B)
hypanthodium
done
clear
C)
spadix
done
clear
D)
verticillaster
done
clear
View Answer play_arrow
question_answer 157) Cladode is a modification of
A)
stem
done
clear
B)
root
done
clear
C)
leaf
done
clear
D)
petiole
done
clear
View Answer play_arrow
question_answer 158) Movement of water through semipermeable membrane produces
A)
wall pressure
done
clear
B)
turgor pressure
done
clear
C)
suction pressure
done
clear
D)
osmotic pressure
done
clear
View Answer play_arrow
question_answer 159) Rate of transpiration is measured by which the following apparatus?
A)
Porometer
done
clear
B)
Respirometer
done
clear
C)
Ganongs potometer
done
clear
D)
Auxanometer
done
clear
View Answer play_arrow
question_answer 160) Which of the following in the site for glycolysis or EMP?
A)
Mitochondria
done
clear
B)
Cytoplasm
done
clear
C)
Nucleus
done
clear
D)
Chloroplast
done
clear
View Answer play_arrow
question_answer 161) In\[{{C}_{4}}\]plants, chloroplast also occurs in
A)
epidermis
done
clear
B)
guard cells
done
clear
C)
spongy parenchyma
done
clear
D)
bundle sheath cells
done
clear
View Answer play_arrow
question_answer 162) Quantasomes occur on the surface of
A)
cristae
done
clear
B)
thylakoids
done
clear
C)
plasmalemma
done
clear
D)
nuclear envelope
done
clear
View Answer play_arrow
question_answer 163) Which is the direction of food through the phloem?
A)
From below upward
done
clear
B)
From tip to bottom
done
clear
C)
From leaves to roots
done
clear
D)
From roots to stem
done
clear
View Answer play_arrow
question_answer 164) Plant cooling is due to
A)
assimilation
done
clear
B)
guttation
done
clear
C)
photorespiration
done
clear
D)
transpiration
done
clear
View Answer play_arrow
question_answer 165) In short day plants, flowering is inhibited by interruption of dark by
A)
white or red light
done
clear
B)
far-red light
done
clear
C)
red light followed by far-red light
done
clear
D)
far red light, then red light and again by far red light
done
clear
View Answer play_arrow
question_answer 166) In angiosperms, triple fusion is required for
A)
embryo
done
clear
B)
endosperm
done
clear
C)
suspensor
done
clear
D)
fruit wall
done
clear
View Answer play_arrow
question_answer 167) Which of the following is the function of tapetum?
A)
Respiratory
done
clear
B)
Nutritive
done
clear
C)
Reproductive
done
clear
D)
Protective
done
clear
View Answer play_arrow
question_answer 168) Which of the following presents premature fall of fruit?
A)
\[G{{A}_{3}}\]
done
clear
B)
Zeatin
done
clear
C)
NAA
done
clear
D)
Ethylene
done
clear
View Answer play_arrow
question_answer 169) Which part of the plant shows thigmotropism?
A)
Leaf lamina
done
clear
B)
Tendrils
done
clear
C)
Root apex
done
clear
D)
Thorns
done
clear
View Answer play_arrow
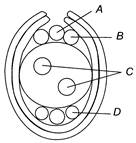
question_answer 170)
Given below is the diagram of a embryo sac. Choose the option in which all the four parts A, B, C and D are correctly identified.
A)
A-Synergids, B-Antipodal cells, C-Egg cell, D-Polar nuclei
done
clear
B)
A-Egg cell, B-Synergids, C-Polar nuclei, D-Antipodal cells
done
clear
C)
A-Egg cell, B-Polar nuclei, C-Synergids, D-Antipodal cells
done
clear
D)
A-Antipodal cells, B-Egg cell, C-Polar nuclei, D-Synergids
done
clear
View Answer play_arrow
question_answer 171) Which of the following pairs of bacteria are generally used in genetic engineering experiments?
A)
Nitrosomonas and Azotobacter
done
clear
B)
Klebsiella and Rhizobium
done
clear
C)
Escherichia and Agrobacterium
done
clear
D)
Diplococcus and Nitrosomonas
done
clear
View Answer play_arrow
question_answer 172) If a heterozygous tall plant is crossed with a homozygous dwarf plant, the proportion of dwarf progeny will be
A)
25%
done
clear
B)
50%
done
clear
C)
75%
done
clear
D)
100%
done
clear
View Answer play_arrow
question_answer 173) The process that involves the transfer of genetic material from one bacterium to another through the agency of bacteriophage is
A)
transformation
done
clear
B)
transcription
done
clear
C)
transduction
done
clear
D)
translocation
done
clear
View Answer play_arrow
question_answer 174) Genes that are involved in turning on or off the transcription of a set of structural genes are called
A)
polymorphic genes
done
clear
B)
operator genes
done
clear
C)
represser genes
done
clear
D)
regulatory genes
done
clear
View Answer play_arrow
question_answer 175) Which of the following enzymes cleaves DNA at specific sites producing sticky end?
A)
Lyases
done
clear
B)
Proteases
done
clear
C)
Restriction endonuclease
done
clear
D)
Cleaving enzyme
done
clear
View Answer play_arrow
question_answer 176) A man, whose father is colourblind, marries a lady who is daughter of a colour blindman. Their off springs will be
A)
all normal
done
clear
B)
all colourblind
done
clear
C)
all sons colourblind
done
clear
D)
some sons colourblind and some normal
done
clear
View Answer play_arrow
question_answer 177) A sedentary sea anemone gets attached to the shell lining of hermit crab. The association is called
A)
symbiosis
done
clear
B)
commensalism
done
clear
C)
parasitism
done
clear
D)
ectoparasitism
done
clear
View Answer play_arrow
question_answer 178) Pseudomonas is an important component of nitrogen cycle. It
A)
fixes elemental nitrogen
done
clear
B)
produces elemental nitrogen
done
clear
C)
transfers elemental nitrogen
done
clear
D)
changes ammonium nitrogen to nitrate state
done
clear
View Answer play_arrow
question_answer 179) Biochemical oxygen demand measures
A)
air pollution
done
clear
B)
industrial pollution
done
clear
C)
pollution capacity of effluents
done
clear
D)
dissolved oxygen needed by microbes to decompost wastes
done
clear
View Answer play_arrow
question_answer 180) The most common indicator organism which represents polluted water is
A)
S. typhi
done
clear
B)
Vibrio cholerae
done
clear
C)
E. coli
done
clear
D)
Entamoeba histolytica
done
clear
View Answer play_arrow
question_answer 181) Submerged hydrophytes have a well developed
A)
root system
done
clear
B)
aerenchyma
done
clear
C)
stomata
done
clear
D)
vascular system
done
clear
View Answer play_arrow
question_answer 182) Dominant species represents most abundant
A)
first tree
done
clear
B)
shrub that appears for the first time
done
clear
C)
herb that binds the soil and provides organic matter to it
done
clear
D)
species having major effect on physical environment
done
clear
View Answer play_arrow
question_answer 183) The mammals (animals) from colder climate generally have shorter hair and not fully developed ear, eyes and other phenotypic characters. This known as
A)
Dalls law
done
clear
B)
Aliens rule
done
clear
C)
Copes law
done
clear
D)
Bergmanns law
done
clear
View Answer play_arrow
question_answer 184) Which of the following is the causative organism of influenza?
A)
Salmonella
done
clear
B)
Streptococcus
done
clear
C)
Myxovirus influenza
done
clear
D)
Rhinovirus
done
clear
View Answer play_arrow
question_answer 185) Addiction of LSD leads to
A)
hallucination
done
clear
B)
damage to kidneys
done
clear
C)
damage to lungs
done
clear
D)
mental and emotional disturbances
done
clear
View Answer play_arrow
question_answer 186) A disease sometimes found in persons above 40 years of age and is characterized by poor. GNS coordination, forgetfulness and tremor of hands is
A)
epilepsy
done
clear
B)
alzheimers disease
done
clear
C)
migraine
done
clear
D)
schizophrenia
done
clear
View Answer play_arrow
question_answer 187) Which of the following is an auto-immune disease?
A)
AIDS
done
clear
B)
Haemophilia
done
clear
C)
Allergy
done
clear
D)
Rheumatoid arthritis
done
clear
View Answer play_arrow
question_answer 188) Taxon is the unit of
A)
species
done
clear
B)
order
done
clear
C)
genus
done
clear
D)
taxonomy
done
clear
View Answer play_arrow
question_answer 189) A virus can be considered a living organism because it
A)
respires
done
clear
B)
responds to touch stimuli
done
clear
C)
can cause disease
done
clear
D)
reproduces (inside the host)
done
clear
View Answer play_arrow
question_answer 190) The basic unit or the lowest taxonomic category is
A)
species
done
clear
B)
family
done
clear
C)
order
done
clear
D)
kingdom
done
clear
View Answer play_arrow
question_answer 191) The excretory organ of cockroach and other insects are
A)
nephridia
done
clear
B)
gizzard
done
clear
C)
flame cells
done
clear
D)
malpighian tubules
done
clear
View Answer play_arrow
question_answer 192) The clitellum of Pheretima is present in segments
A)
12, 13 and 14
done
clear
B)
13, 14 and 15
done
clear
C)
14, 15 and 16
done
clear
D)
15, 16 and 17
done
clear
View Answer play_arrow
question_answer 193) Which of the following is a loose connective tissue?
A)
Adipose tissue
done
clear
B)
Areolar tissue
done
clear
C)
Blood
done
clear
D)
Nervous tissue
done
clear
View Answer play_arrow
question_answer 194) Where does the synthesis of ATP in mitochondria takes place?
A)
In matrix
done
clear
B)
In intercristal space
done
clear
C)
Upon cristae
done
clear
D)
At outer membrane
done
clear
View Answer play_arrow
question_answer 195) Golgi apparatus is specialised for
A)
energy transduction
done
clear
B)
digestion of protein
done
clear
C)
glycosidation of proteins and lipids
done
clear
D)
digestion of carbohydrates
done
clear
View Answer play_arrow
question_answer 196) Exchange of paternal and maternal chromosomes material during cell division is called
A)
dyad formation
done
clear
B)
bivalent formation
done
clear
C)
synapsis
done
clear
D)
crossing over
done
clear
View Answer play_arrow
question_answer 197) In mitosis, where does the chromosome duplication occur?
A)
Interphase
done
clear
B)
Prophase
done
clear
C)
Late prophase
done
clear
D)
Latetelophase
done
clear
View Answer play_arrow
question_answer 198) Linoleic acid is an unsaturated fatty acid and its content is highest in
A)
coconut oil
done
clear
B)
sunflower oil
done
clear
C)
groundnut oil
done
clear
D)
cotton oil
done
clear
View Answer play_arrow
question_answer 199) The product of an enzyme-catalysed reaction can act as an inhibitor of the reaction. This mechanism of control is known as
A)
feed back inhibition
done
clear
B)
competitive inhibition
done
clear
C)
represssion
done
clear
D)
non-competitive inhibition
done
clear
View Answer play_arrow
question_answer 200) The respiratory centre in brain is stimulated by
A)
\[C{{O}_{2}}\]concentration in venous blood
done
clear
B)
\[{{O}_{2}}\]concentration in venous blood
done
clear
C)
\[{{O}_{2}}\]concentration is arterial blood
done
clear
D)
\[C{{O}_{2}}\]concentration in arterial blood
done
clear
View Answer play_arrow
question_answer 201) Glucose present in glomerular filterate is reabsorbed in
A)
Bowmans capsule
done
clear
B)
Henles loop
done
clear
C)
proximal convoluted tubule
done
clear
D)
distal convoluted tubule
done
clear
View Answer play_arrow
question_answer 202) Which is the largest bone in middle ear?
A)
Incus
done
clear
B)
Malleus
done
clear
C)
Stapes
done
clear
D)
Cochlea
done
clear
View Answer play_arrow
question_answer 203) Which of the following activities in disturbed, if parathyroid gland degenerates?
A)
Growth
done
clear
B)
Sodium concentration
done
clear
C)
Potassium concentration
done
clear
D)
Calcium concentration
done
clear
View Answer play_arrow
question_answer 204) Retina is composed of
A)
rods only
done
clear
B)
cones only
done
clear
C)
rods and cones
done
clear
D)
rods, cones and neuroganglion cells
done
clear
View Answer play_arrow
question_answer 205) Which of the following vertebrate organs receives only oxygenated blood?
A)
Gill
done
clear
B)
Lung
done
clear
C)
Liver
done
clear
D)
Spleen
done
clear
View Answer play_arrow
question_answer 206) Which of the following is agranulocyte?
A)
Lymphocyte
done
clear
B)
Eosinophill
done
clear
C)
Basophill
done
clear
D)
Neutrophill
done
clear
View Answer play_arrow
question_answer 207) The rate of heart beat is determined by
A)
SA node
done
clear
B)
AV node
done
clear
C)
Purkinje fibre
done
clear
D)
Papillary muscles
done
clear
View Answer play_arrow
question_answer 208) The middle piece of the sperm contains
A)
centriole
done
clear
B)
nucleus
done
clear
C)
protein
done
clear
D)
mitochondria
done
clear
View Answer play_arrow
question_answer 209) Secretion of progesterone by corpus luteum is initiated by
A)
Gh
done
clear
B)
LH
done
clear
C)
thyroxine
done
clear
D)
testosterone
done
clear
View Answer play_arrow
question_answer 210) The function of oxytocin is to help in
A)
child birth
done
clear
B)
growth
done
clear
C)
lactation
done
clear
D)
gametogenesis
done
clear
View Answer play_arrow
question_answer 211) Human gametes differ from all other body cells as they are
A)
motile
done
clear
B)
diploid
done
clear
C)
haploid
done
clear
D)
without cell wall
done
clear
View Answer play_arrow
question_answer 212) Which one of the following is initiated by secretion of trophoblast?
A)
Blastulation
done
clear
B)
Gastrulation
done
clear
C)
Implantation
done
clear
D)
Cleavage
done
clear
View Answer play_arrow
question_answer 213) Spermatogenesis changes
A)
spermatogonium to primary spermatocyte
done
clear
B)
primary spermatocyte to secondary spermatocyte
done
clear
C)
secondary spermatocyte to spermatid
done
clear
D)
spermatid to spermatozoa
done
clear
View Answer play_arrow
question_answer 214) Which of the following is a hormone releasing IUD?
A)
Multiload375
done
clear
B)
Cu-7
done
clear
C)
LNG-20
done
clear
D)
CuT
done
clear
View Answer play_arrow
question_answer 215) Plasmids present in the bacterial cells are
A)
linear double helical RNA molecules
done
clear
B)
linear double helical DNA molecules
done
clear
C)
circular double helical DNA molecules
done
clear
D)
circular double helical RNA molecules
done
clear
View Answer play_arrow
question_answer 216) In which of the following diseases, the man has an extra X-chromosome?
A)
Bleeders disease
done
clear
B)
Turners syndrome
done
clear
C)
Downs syndrome
done
clear
D)
Klinefelters syndrome
done
clear
View Answer play_arrow
question_answer 217) Which of the following takes place in DNA fingerprinting?
A)
A positive identification can be made
done
clear
B)
Multiple restriction enzyme digests/generates unique fragments
done
clear
C)
The polymerase chain reaction amplifies fewer DNA
done
clear
D)
The verability of repeated sequences between two restriction sites is evaluated
done
clear
View Answer play_arrow
question_answer 218) The scientist related with the theory of biogenesis and who has done experiment with swan-necked flask is
A)
Haeckel
done
clear
B)
Louis Pasteur
done
clear
C)
van Helmont
done
clear
D)
Miller
done
clear
View Answer play_arrow
question_answer 219) A plant hormone used for inducing morphogenesis in plant tissue culture is
A)
ABA
done
clear
B)
gibberellins
done
clear
C)
cytokinins
done
clear
D)
ethylene
done
clear
View Answer play_arrow
question_answer 220) Triticale is obtained by crossing wheat with
A)
oat
done
clear
B)
maize
done
clear
C)
barley
done
clear
D)
rye
done
clear
View Answer play_arrow
question_answer 221) A bio-fertiliser is
A)
a cyanobacteria like Anabaena species living in cavities of Azolla leaves
done
clear
B)
symbiotic association like Azotobacter which fixed atmospheric nitrogen
done
clear
C)
farm yard manure consisting of mixture of cattle dung and crop
done
clear
D)
green manure in which a quickly growing crop is cultivated and ploughed under
done
clear
View Answer play_arrow
question_answer 222) Which one of the following drugs depresses (switch off) the activities of central nervous system, is known as sedative and also gives feeling of calmness, relaxation of drowsiness?
A)
Opium
done
clear
B)
Barbiturate
done
clear
C)
Heroin
done
clear
D)
Cocaine
done
clear
View Answer play_arrow
question_answer 223) Which of the following techniques is used to make numerous copies of a specific segment of DNA quickly and accurately?
A)
Translation
done
clear
B)
Transcription
done
clear
C)
Polymerase chain reaction
done
clear
D)
Ligase chain reaction
done
clear
View Answer play_arrow
question_answer 224) Which of the following recent technique is used for separating fragments of DNA?
A)
Eastern blotting
done
clear
B)
Northern blotting
done
clear
C)
Southern blotting
done
clear
D)
Western blotting
done
clear
View Answer play_arrow
question_answer 225) In case of Bacillus thuringiensis. Bacillus itself is not killed by the toxic protein crystals produced by it because Bt toxin
A)
protein is not produced in the Bacillus
done
clear
B)
cannot causes and damage to Bacillus
done
clear
C)
protein is produced in very less amount in the Bacillus
done
clear
D)
exist as the inactive toxin
done
clear
View Answer play_arrow