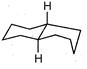
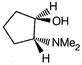
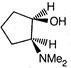
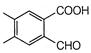
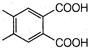
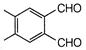
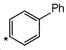
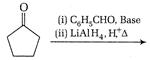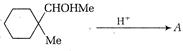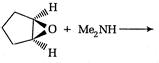question_answer 1) Conductivity of semiconductors
A)
is maximum at\[0\text{ }K\]
done
clear
B)
decreases with increase in temperature
done
clear
C)
increases with increase in temperature
done
clear
D)
is maximum at 300 K
done
clear
View Answer play_arrow
question_answer 2) Two copper spheres having same radii, one solid and other hollow, are charged to the same potential. Which of the following statements is correct?
A)
Hollow sphere will hold more charge
done
clear
B)
Solid sphere will hold more charge
done
clear
C)
Solid sphere will have uniform volume charge density
done
clear
D)
Both spheres will hold same charge
done
clear
View Answer play_arrow
question_answer 3) Two pendula oscillate with a constant phase difference of\[45{}^\circ \]and same amplitude. If the maximum velocity of one of them is v and that of other is\[v+x,\]then the value of\[x\]will be
A)
0
done
clear
B)
\[v/2\]
done
clear
C)
\[v/\sqrt{2}\]
done
clear
D)
\[(\sqrt{2})v\]
done
clear
View Answer play_arrow
question_answer 4) An observer standing near the sea-coast counts 48 waves per min. If the wavelength of the wave is 10 m, the velocity of the waves will be
A)
8m/s
done
clear
B)
12m/s
done
clear
C)
16m/s
done
clear
D)
20m/s
done
clear
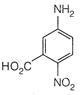
View Answer play_arrow
question_answer 5) The carbon resistor has the color band sequence of green, orange, blue and silver. The value of resistance will be
A)
\[64\times {{10}^{7}}\pm 20%\,\Omega \]
done
clear
B)
\[53\times {{10}^{6}}\pm 20%\,\Omega \]
done
clear
C)
\[64\times {{10}^{7}}\pm 10%\,\Omega \]
done
clear
D)
\[53\times {{10}^{6}}\pm 10%\,\Omega \]
done
clear
View Answer play_arrow
question_answer 6) A parallel narrow-beam of light is falling normally on a glass sphere. It will come to a focus
A)
inside the sphere (except at its centre)
done
clear
B)
on the surface of the sphere
done
clear
C)
outside the sphere
done
clear
D)
exactly at the centre of the sphere
done
clear
View Answer play_arrow
question_answer 7) Which of the following is NOT an electromagnetic wave?
A)
Sound wave
done
clear
B)
Thermal radiation
done
clear
C)
Microwave
done
clear
D)
Gamma ray
done
clear
View Answer play_arrow
question_answer 8) The ratio of mass defect of the nucleus to its mass number is maximum for
A)
\[{{U}^{238}}\]
done
clear
B)
\[{{N}^{14}}\]
done
clear
C)
\[S{{i}^{28}}\]
done
clear
D)
\[F{{e}^{56}}\]
done
clear
View Answer play_arrow
question_answer 9) Assuming density d of a planet to be uniform, we can say that the time period of its artificial satellite is proportional to
A)
\[d\]
done
clear
B)
\[\sqrt{d}\]
done
clear
C)
\[1/\sqrt{d}\]
done
clear
D)
\[1/d\]
done
clear
View Answer play_arrow
question_answer 10) An ideal gas is heated at constant volume until its pressure doubles. Which one of the following statements is correct?
A)
The mean speed of the molecules doubles
done
clear
B)
Root mean square speed of the molecules doubles
done
clear
C)
Mean square speed of the molecules doubles
done
clear
D)
Mean square speed of the molecules remains unchanged
done
clear
View Answer play_arrow
question_answer 11) In a cyclic process, the change in the internal energy of a system over one complete cycle
A)
depends on the path
done
clear
B)
is always negative
done
clear
C)
is always zero
done
clear
D)
is always positive
done
clear
View Answer play_arrow
question_answer 12) In a transformer the number of primary turns is four times that of the secondary turns. Its primary is connected to an AC source of voltage V. Then,
A)
current through its secondary is about four times that of the current through its primary
done
clear
B)
voltage across its secondary is about four times that of the voltage across its primary
done
clear
C)
voltage across its secondary is about two times that of the voltage across its primary
done
clear
D)
voltage across its secondary is about\[1/(2\sqrt{2})\]times that of the voltage across its primary
done
clear
View Answer play_arrow
question_answer 13) The path of a charge particle after it enters a region of a uniform electrostatic field with velocity perpendicular to the field will be
A)
straight line
done
clear
B)
circular
done
clear
C)
helical
done
clear
D)
parabolic
done
clear
View Answer play_arrow
question_answer 14) The dimension of magnetic flux is
A)
\[[ML{{T}^{-1}}{{A}^{-1}}]\]
done
clear
B)
\[[M{{L}^{-1}}T{{A}^{-2}}]\]
done
clear
C)
\[[M{{L}^{-2}}{{T}^{2}}{{A}^{-2}}]\]
done
clear
D)
\[[M{{L}^{2}}{{T}^{-2}}{{A}^{-1}}]\]
done
clear
View Answer play_arrow
question_answer 15) A ball is dropped from the top of 80 m high tower. If after 2 s of fall the gravity\[(g=10\text{ }m/{{s}^{2}})\]disappears, then time taken to reach the ground since the gravity disappeared is
A)
2s
done
clear
B)
3s
done
clear
C)
4s
done
clear
D)
5s
done
clear
View Answer play_arrow
question_answer 16) Which of the following is correct statement about the magnitude of the acceleration a of the particle executing simple harmonic motion?
A)
a will be maximum at the equilibrium position .
done
clear
B)
a will be maximum at the extreme position
done
clear
C)
a will be always constant
done
clear
D)
a will always be zero
done
clear
View Answer play_arrow
question_answer 17) A concave mirror has focal length\[f\]. A convergent beam of light is made incident on it. Then the image distance\[V\]is
A)
zero
done
clear
B)
less than\[f\]
done
clear
C)
equal to\[f\]
done
clear
D)
more than\[f\]
done
clear
View Answer play_arrow
question_answer 18) In an n-p-n transistor, p is
A)
intrinsic semiconductor
done
clear
B)
emitter
done
clear
C)
collector
done
clear
D)
base
done
clear
View Answer play_arrow
question_answer 19) In the fringe pattern of a Youngs double slit experiment the ratio of intensities of maxima and minima is 25 : 9. Then, the ratio of the amplitudes of interfering waves is
A)
\[4:1\]
done
clear
B)
\[5:3\]
done
clear
C)
\[4:3\]
done
clear
D)
\[25:9\]
done
clear
View Answer play_arrow
question_answer 20) Consider a ray of light travelling from a denser to a rarer medium. If it is incident at the critical angle then
A)
it will emerge out into the rarer medium
done
clear
B)
it will undergo total internal reflection
done
clear
C)
it will travel along the interface separating the two media
done
clear
D)
it will retrace its path
done
clear
View Answer play_arrow
question_answer 21) Radius of Earth is 6400 km and that of Mars is 3200 km. Mass of Mars is 0.1 that of Earths mass. Then, the acceleration due to gravity on Mars is nearly
A)
\[1\text{ }m/{{s}^{2}}\]
done
clear
B)
\[\text{2}\text{.5 }m/{{s}^{2}}\]
done
clear
C)
\[\text{4 }m/{{s}^{2}}\]
done
clear
D)
\[\text{5 }m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 22) Consider boiling water converting into steam. Under this condition, the specific heat of water is
A)
less than zero
done
clear
B)
zero
done
clear
C)
slightly greater than zero
done
clear
D)
infinite
done
clear
View Answer play_arrow
question_answer 23) Smallest division on the main scale of given vernier calipers is 0.5 mm. Vernier scale has 2 5 divisions and these coincide with 24 main scale divisions. The least count of vernier calipers is
A)
0.001 cm
done
clear
B)
0.002cm
done
clear
C)
0.01 cm
done
clear
D)
0.02cm
done
clear
View Answer play_arrow
question_answer 24) If R is Rydbergs constant, the series limit of the wavelength of Balmer series for hydrogen atom is given by
A)
\[1/R\]
done
clear
B)
\[4/R\]
done
clear
C)
\[9/R\]
done
clear
D)
\[16/R\]
done
clear
View Answer play_arrow
question_answer 25) In which of the following both transverse and longitudinal waves propagate?
A)
Heat transfer
done
clear
B)
Elastic wave motion in a solid
done
clear
C)
Microwave communication
done
clear
D)
X-ray motion
done
clear
View Answer play_arrow
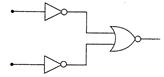
question_answer 26)
The combination of gates as shown in the figure forms the
A)
AND gate
done
clear
B)
OR gale
done
clear
C)
NOR gate
done
clear
D)
NOT gate
done
clear
View Answer play_arrow
question_answer 27) A magnet makes a single pass through a coil. Then, across the ends of the coil it produces
A)
DC voltage
done
clear
B)
sinusoidal voltage
done
clear
C)
single voltage pulse
done
clear
D)
two voltage pulses
done
clear
View Answer play_arrow
question_answer 28) The energy per mole per degree of freedom of an ideal gas is
A)
\[(3/2){{k}_{B}}T\]
done
clear
B)
\[(1/2){{k}_{B}}T\]
done
clear
C)
\[(3/2)RT\]
done
clear
D)
\[(1/2)RT\]
done
clear
View Answer play_arrow
question_answer 29) Metal alloys are used for making standard resistance coils because
A)
they have high thermal conductivity
done
clear
B)
their resistance depends weakly on temperature
done
clear
C)
they have low thermal conductivity
done
clear
D)
their resistance depends strongly on temperature
done
clear
View Answer play_arrow
question_answer 30) The dielectric constant of a perfect conductor is
A)
+1
done
clear
B)
0
done
clear
C)
infinite
done
clear
D)
\[-1\]
done
clear
View Answer play_arrow
question_answer 31) The 220 V AC line voltage that we receive in our homes is
A)
rms value
done
clear
B)
peak value
done
clear
C)
average value
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 32) A person is standing on a weighing-scale and observes that the reading is 60 kg. He then suddenly jumps up and observes that reading goes to 70 kg. Then his maximum upward acceleration is
A)
zero
done
clear
B)
\[1.4\text{ }m/{{s}^{2}}\]
done
clear
C)
\[1.63\text{ }m/{{s}^{2}}\]
done
clear
D)
\[9.8\text{ }m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 33) A solid sphere is rolling down an inclined plane. Then, the ratio of its translational kinetic energy to its rotational kinetic energy is
A)
2.5
done
clear
B)
1.5
done
clear
C)
1
done
clear
D)
0.4
done
clear
View Answer play_arrow
question_answer 34) A block of mass 3 kg starts from rest and slides down a curved path in the shape of a quarter-circle of radius 2 m and reaches the bottom of path with a speed 1 m/s. If g is\[10\text{ }m/{{s}^{2}},\]the amount of work done against friction is
A)
60J
done
clear
B)
36J
done
clear
C)
24J
done
clear
D)
12J
done
clear
View Answer play_arrow
question_answer 35) Values for Brewsters angle can be
A)
only less than \[45{}^\circ \]
done
clear
B)
only greater than\[45{}^\circ \]
done
clear
C)
any value in the range\[0{}^\circ \]to\[90{}^\circ \]except\[45{}^\circ \]
done
clear
D)
any value in the range\[0{}^\circ \]to\[90{}^\circ \]including\[45{}^\circ \]
done
clear
View Answer play_arrow
question_answer 36) Consider a bi-convex lens and a plano-convex lens, with radii of curvature of all the curved surfaces being same. If /is the focal length of bi-convex lens then, the focal length of the plano-convex lens is
A)
\[4f\]
done
clear
B)
\[2f\]
done
clear
C)
\[f\]
done
clear
D)
\[0.5f\]
done
clear
View Answer play_arrow
question_answer 37) A body is travelling towards East with a speed of 9 m/s and with an acceleration of\[2\text{ }m/{{s}^{2}}\]acting along West on it. The displacement of the body during the 5th second of its motion is
A)
0.25 m
done
clear
B)
0.5 m
done
clear
C)
0.75 m
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 38) Bulk modulus is defined by
A)
increase in length per unit length per unit applied stress
done
clear
B)
increase in volume per unit volume per unit applied stress
done
clear
C)
lateral displacement per unit length per unit applied stress
done
clear
D)
change in cross-sectional area per unit area per unit applied stress
done
clear
View Answer play_arrow
question_answer 39) A bullet fired from a rifle loses 20% of its speed while passing through a wooden plank. Then, minimum number of wooden planks required to completely stop the bullet is
A)
3
done
clear
B)
5
done
clear
C)
15
done
clear
D)
25
done
clear
View Answer play_arrow
question_answer 40) If the forward bias voltage in a p-n junction diode is decreased, the length of depletion region will
A)
increase
done
clear
B)
decrease
done
clear
C)
not change
done
clear
D)
initially increase and then decrease
done
clear
View Answer play_arrow
question_answer 41) A particle is undergoing uniform circular motion with angular momentum L. While moving on the same path if its kinetic energy becomes four times, then its angular momentum will be
A)
L/4
done
clear
B)
L/2
done
clear
C)
L
done
clear
D)
2L
done
clear
View Answer play_arrow
question_answer 42) A 1m long solenoid containing 1000 turns produces a flux density of\[3.14\times {{10}^{-3}}T\]. The current in the solenoid will be
A)
2.0 A
done
clear
B)
2.5 A
done
clear
C)
3.0 A
done
clear
D)
3.5 A
done
clear
View Answer play_arrow
question_answer 43) Which of the following is incorrect about sky waves?
A)
Sky waves are not used in long distance communication
done
clear
B)
Their propagation takes place by total internal reflection
done
clear
C)
Sky waves support the so-called AM band
done
clear
D)
The frequency of sky waves ranges typically from 3 MHz to 30 MHz
done
clear
View Answer play_arrow
question_answer 44) The wave nature of electrons is demonstrated by the
A)
Photoelectric effect
done
clear
B)
Rutherfords experiment
done
clear
C)
Dopplers effect
done
clear
D)
Davisson and Germer experiment
done
clear
View Answer play_arrow
question_answer 45) A person carrying a. whistle emitting continuously a note of 272 Hz is running towards a reflecting surface with a speed of 18 km/h. If the speed of sound is 345 m/s, the number of beats heard by him are
A)
4
done
clear
B)
6
done
clear
C)
8
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 46)
Consider the two cells having emf\[{{E}_{1}}\]and\[{{E}_{2}}\]\[({{E}_{1}}>{{E}_{2}})\]connected as shown in the figure. A potentiometer is used to measure potential difference between P and Q and the balancing length of the potentiometer wire is 0.8 m. Same potentiometer is then used to measure potential difference between P and R and the balancing length is 0.2 m. Then, the ratio\[{{E}_{1}}/{{E}_{2}}\]is
A)
4/3
done
clear
B)
5/4
done
clear
C)
5/3
done
clear
D)
4/1
done
clear
View Answer play_arrow
question_answer 47) Which of the following is NOT an example of primary cell?
A)
Voltaic cell
done
clear
B)
Lead-acid cell
done
clear
C)
Daniel cell
done
clear
D)
Leclanche cell
done
clear
View Answer play_arrow
question_answer 48) Red, blue, green and violet colour lights are one by one made incident on a photocathode. It is observed that only one color light produces photo-electrons.
A)
Red
done
clear
B)
Blue
done
clear
C)
Green
done
clear
D)
Violet
done
clear
View Answer play_arrow
question_answer 49) What amount of original radioactive material is left?
A)
6.5%
done
clear
B)
12.5%
done
clear
C)
25.5%
done
clear
D)
33.3%
done
clear
View Answer play_arrow
question_answer 50) Consider a region of uniform magnetic field directed along positive X-axis. Now a positive test charge Q, located at origin 0 (0, 0) inside the field, is released from rest position. The particle will
A)
remain stationary at origin\[O\]
done
clear
B)
move along positive X-axis
done
clear
C)
move along negative X-axis
done
clear
D)
undergo a circular motion in the\[X-Y\]plane
done
clear
View Answer play_arrow
question_answer 51) A charge particle having charge\[1\times {{10}^{-19}}C\]revolves in an orbit of radius\[1\overset{o}{\mathop{A}}\,\]such that the frequency of revolution is\[{{10}^{16}}Hz\]. The resulting magnetic moment in SI units will be
A)
\[1.57\times {{10}^{-21}}\]
done
clear
B)
\[3.14\times {{10}^{-21}}\]
done
clear
C)
\[1.57\times {{10}^{-23}}\]
done
clear
D)
\[3.14\times {{10}^{-23}}\]
done
clear
View Answer play_arrow
question_answer 52) The length of antenna to transmit waves of 1 MHz will be
A)
3m
done
clear
B)
15 m
done
clear
C)
30m
done
clear
D)
300m
done
clear
View Answer play_arrow
question_answer 53) A series LCR circuit is connected to an AC source and is showing resonance. Then
A)
\[{{V}_{R}}=0\]
done
clear
B)
\[{{V}_{L}}={{V}_{R}}\]
done
clear
C)
\[{{V}_{C}}={{V}_{R}}\]
done
clear
D)
\[{{V}_{L}}={{V}_{C}}\]
done
clear
View Answer play_arrow
question_answer 54) Dimensions of Plancks constant are
A)
\[[M{{L}^{2}}{{T}^{-1}}]\]
done
clear
B)
\[[M{{L}^{2}}{{T}^{-3}}]\]
done
clear
C)
\[[ML{{T}^{-1}}]\]
done
clear
D)
\[[M{{L}^{3}}{{T}^{-3}}]\]
done
clear
View Answer play_arrow
question_answer 55) Consider an electric dipole placed in a region of non-uniform electric field. Choose the correct statement out of the following options:
A)
The dipole will experience only a force
done
clear
B)
The dipole will experience only a torque
done
clear
C)
The dipole will experience both the force and torque
done
clear
D)
The dipole will neither experience a force nor a torque
done
clear
View Answer play_arrow
question_answer 56) A block of mass m is placed on an inclined plane having coefficient of friction W. The plane is making an angle 0 with the horizontal. The minimum value of upward force acting along the inclined plane that can just move the block up is
A)
\[mg\,\cos \theta \]
done
clear
B)
\[mmg\,\cos \theta \]
done
clear
C)
\[mg\,\sin \theta \]
done
clear
D)
\[mmg\,\sin \theta \]
done
clear
View Answer play_arrow
question_answer 57) A ball is projected up at an angle\[\theta \]with horizontal from the top of a tower with speed V. It hits the ground at point A after time\[{{t}_{A}}\]with speed\[{{V}_{A}}\]. Now, this ball is projected at same angle and speed from the base of the tower (located at point P) and it hits ground at point B after time\[{{t}_{B}}\]with speed\[{{V}_{B}}\]. Then
A)
\[PA=PB\]
done
clear
B)
\[{{t}_{A}}={{t}_{B}}\]
done
clear
C)
\[{{V}_{A}}>{{V}_{B}}\]
done
clear
D)
ball A hits the ground at an angle\[(-\theta )\]with horizontal
done
clear
View Answer play_arrow
question_answer 58) The electric field of an electric dipole at a point on its axis at a distance\[d\]from the centre of the dipole varies as
A)
\[1/d\]
done
clear
B)
\[1/{{d}^{2}}\]
done
clear
C)
\[1/{{d}^{3}}\]
done
clear
D)
\[1/{{d}^{3/2}}\]
done
clear
View Answer play_arrow
question_answer 59) Un-polarised light is travelling from a medium of refractive index 2 to a medium of refractive index 3. The angle of incidence is\[60{}^\circ \]. Then
A)
Reflected light will be partially polarized
done
clear
B)
reflected/light will be plane polarised in a plane perpendicular to plane of incidence
done
clear
C)
refracted light will be plane polarised in a plane perpendicular to plane of incidence
done
clear
D)
refracted light will be plane polarised in a plane parallel to plane of incidence
done
clear
View Answer play_arrow
question_answer 60) Newtons law of cooling applies when a body is losing heat to its surroundings by
A)
conduction
done
clear
B)
convection
done
clear
C)
radiation
done
clear
D)
conduction as well as radiation
done
clear
View Answer play_arrow
question_answer 61) If a homogeneous colloid placed in dark is observed in the direction of light, it appears clear and if it is observed from a direction at right angles to the direction of light beam, it appears perfectly dark. This is known as
A)
Brownian effect
done
clear
B)
Hardy Schulze effect
done
clear
C)
Einstein effect
done
clear
D)
Tyndall effect
done
clear
View Answer play_arrow
question_answer 62) When powdered plaster of Paris is mixed with correct amount of water, it sets into a solid mass of
A)
\[CaS{{O}_{4}}.5{{H}_{2}}O\]
done
clear
B)
\[CaS{{O}_{4}}.3{{H}_{2}}O\]
done
clear
C)
\[CaS{{O}_{4}}.2{{H}_{2}}O\]
done
clear
D)
\[CaS{{O}_{4}}.1/2{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 63) \[PV\]value decreases with increases in p at constant temperature when
A)
there is no attractive or repulsive forces between molecules
done
clear
B)
attractive and repulsive forces between molecules are equal
done
clear
C)
attractive forces between molecules are predominant
done
clear
D)
repulsive forces between molecules are predominant
done
clear
View Answer play_arrow
question_answer 64) For a reaction,\[C(s)+C{{O}_{2}}(g)\xrightarrow{{}}2CO(g);\]the partial pressure of\[C{{O}_{2}}\]and\[CO\]are 4 and 8 atm, respectively.\[{{K}_{p}}\]for the reaction is
A)
0.5
done
clear
B)
2
done
clear
C)
16
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 65) HA is a weak acid. At\[25{}^\circ C,\]the molar conductivity of 0.02 M HA is\[150\,{{\Omega }^{-1}}c{{m}^{2}}mo{{l}^{-1}}.\]If its\[\Lambda _{m}^{o}\]is\[300\,{{\Omega }^{-1}}c{{m}^{2}}mo{{l}^{-1}},\]then equilibrium constant of HA dissociation is
A)
0.001
done
clear
B)
0.005
done
clear
C)
0.01
done
clear
D)
0.02
done
clear
View Answer play_arrow
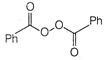
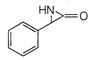
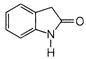
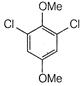
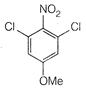
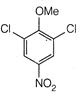
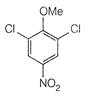
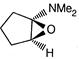
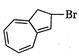
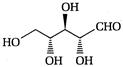
question_answer 66)
The product of the following reaction is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
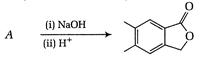
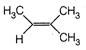
question_answer 67)
Identify the major product for the reaction given below:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 68) 30 mL of 0.02 M ammonium hydroxide is mixed with 15 mL of\[0.02\text{ }M\text{ }HCl\]. What will be the pH of the solution\[(p{{K}_{b}}=4.0)\]?
A)
4
done
clear
B)
8
done
clear
C)
4
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 69) In an acidified aqueous solution of\[M{{n}^{2+}},N{{i}^{2+}},C{{u}^{2+}}\] and\[H{{g}^{2+}}\]ions,\[{{H}_{2}}S\]gas was passed. Precipitates are
A)
MnS and CuS
done
clear
B)
NiS and HgS
done
clear
C)
MnS and NiS
done
clear
D)
CuS and HgS
done
clear
View Answer play_arrow
question_answer 70) Shape of\[S{{F}_{4}}\]is
A)
tetrahedral
done
clear
B)
square planar
done
clear
C)
trigonal pyramid
done
clear
D)
see-saw
done
clear
View Answer play_arrow
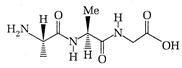
question_answer 71)
The following tripeptide can be synthesized from the following ammo acid
A)
Glycine, Leucine and Alanine
done
clear
B)
Alanine, Isoleucine and Giycine
done
clear
C)
Valine, Alanine and Glycine
done
clear
D)
Alanine, Serine and Glycine
done
clear
View Answer play_arrow
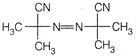
question_answer 72) The species which cannot serve as an initiator for the free radical polymerisation, is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 73) Nylon is a
A)
polyamide
done
clear
B)
carbonate
done
clear
C)
ester
done
clear
D)
polycarboxylic acid
done
clear
View Answer play_arrow
question_answer 74) A gas at high temperature is cooled. The highest temperature at which liquefaction of gas first occurs is called
A)
Boyle temperature
done
clear
B)
critical temperature
done
clear
C)
boiling temperature
done
clear
D)
freezing temperature
done
clear
View Answer play_arrow
question_answer 75) Buna-N synthetic rubber is obtained by copolyrnerisation of
A)
\[C{{H}_{2}}=CH-CH=C{{H}_{2}}\]and \[{{H}_{5}}{{C}_{6}}-CH=C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{2}}=CH-CN\]and \[{{H}_{2}}C=CH-CH=C{{H}_{2}}\]
done
clear
C)
\[{{H}_{2}}C=CH-CN\]and \[C{{H}_{2}}=CH-C(C{{H}_{3}})=C{{H}_{2}}\]
done
clear
D)
\[{{H}_{2}}C=CH-C(Cl)=C{{H}_{2}}\]and \[{{H}_{2}}C=CH-CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
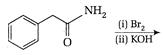
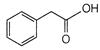
question_answer 76)
When the following amide is treated with \[B{{i}_{2}}/KOH,\]it gives
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 77) The IUPAC name of the coordination compound\[[Co{{({{H}_{2}}O)}_{2}}{{(N{{H}_{3}})}_{4}}]C{{l}_{3}}\]is
A)
tetraamminediaquacobalt (III) chloride
done
clear
B)
cobalt (III) tetraamminediaqua chloride
done
clear
C)
diaquatetraammine cobalt (III) chloride
done
clear
D)
tetraamminediaquacobalt (II) chloride
done
clear
View Answer play_arrow
question_answer 78) Siderite is mainly ore of
A)
Zn
done
clear
B)
Fe
done
clear
C)
Cd
done
clear
D)
Ru
done
clear
View Answer play_arrow
question_answer 79) Among\[P,S,Cl,F,\]the elements with most negative and least negative electron gain enthalpy respectively, are
A)
\[Cl,S\]
done
clear
B)
\[F,S\]
done
clear
C)
\[Cl,P\]
done
clear
D)
\[F,P\]
done
clear
View Answer play_arrow
question_answer 80) For a reaction\[2A\xrightarrow[{}]{{}}3B;\]if the rate of formation of B is\[x\text{ }mol/L,\]the rate of consumption of A is
A)
\[x\]
done
clear
B)
\[\frac{3x}{2}\]
done
clear
C)
\[\frac{2x}{3}\]
done
clear
D)
\[3x\]
done
clear
View Answer play_arrow
question_answer 81)
The following alcohol after treatment with acid gives compound A. Ozonolsis of A gives nonane-2, 8-dione. The compound A is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 82) In\[H{{e}_{2}},\]the electrons in bonding and anti-bonding orbitals are
A)
2, 2
done
clear
B)
4, 2
done
clear
C)
4, 0
done
clear
D)
2, 4
done
clear
View Answer play_arrow
question_answer 83) 0.5 molal solution of a solute in benzene shows a depression in freezing point equal to 2 K. Molal depression constant for benzene is\[5\text{ }K\text{ }kg\text{ }mo{{l}^{-1}}\]. If the solute forms dimer in benzene, what is the % association?
A)
40
done
clear
B)
50
done
clear
C)
60
done
clear
D)
80
done
clear
View Answer play_arrow
question_answer 84)
The major product of the following transformation is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 85)
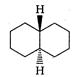
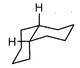
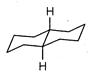
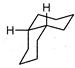
A highly stable conformation for the following compound is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 86) Molar enthalpy change for melting of ice is 6 kJ/mol. Then the internal energy change (in kJ/mol) when 1 mole of water is converted into ice at 1 atm at\[0{}^\circ C\]is
A)
\[RT/1000\]
done
clear
B)
\[6\]
done
clear
C)
\[6-(RT/1000)\]
done
clear
D)
\[6+(RT/1000)\]
done
clear
View Answer play_arrow
question_answer 87)
Identify the correct product of the following reaction
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 88) Among second period elements, the correct order for first ionisation enthalpy is
A)
\[Li<Be<B<C<N<O<F<Ne\]
done
clear
B)
\[Li<B<Be<C<O<N<F<Ne\]
done
clear
C)
\[Li>Be>B>C>N>O>F>Ne\]
done
clear
D)
\[Li>B>C>Be>O>N>F>Ne\]
done
clear
View Answer play_arrow
question_answer 89) For the reaction \[A(s)+2{{B}^{+}}(aq)\to {{A}^{2+}}(aq)+2B(s);\] the\[E{}^\circ \]is 1.18 V. Then the equilibrium constant for the reaction is
A)
1010
done
clear
B)
1020
done
clear
C)
1040
done
clear
D)
1060
done
clear
View Answer play_arrow
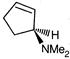
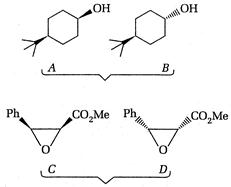
question_answer 90)
Identify the correct statement about the following pairs of compounds.
A)
A and B diastereomer; C and D diastereomer
done
clear
B)
A and B enantiomer; C and D diastereomer
done
clear
C)
A and B diastereomer; C and D enantiomer
done
clear
D)
A and B enantiomer; C and D enantiomer
done
clear
View Answer play_arrow
question_answer 91) One of the following complexes shows geometrical isomerism. The complex is
A)
\[PtC{{l}_{4}}\]
done
clear
B)
\[Pt{{(N{{H}_{3}})}_{2}}C{{l}_{2}}\]
done
clear
C)
\[Pt{{(N{{H}_{3}})}_{3}}Cl\]
done
clear
D)
\[Ni{{(N{{H}_{3}})}_{3}}Cl\]
done
clear
View Answer play_arrow
question_answer 92) The ionisation potential of hydrogen atom is 13.6 eV. The energy required to remove an electron from\[n=2\]state of hydrogen atom is
A)
27.2eV
done
clear
B)
13.6eV
done
clear
C)
6.8eV
done
clear
D)
3.4eV
done
clear
View Answer play_arrow
question_answer 93) Among the following pair, which one has both variables as intensive variable?
A)
T, V
done
clear
B)
m, p
done
clear
C)
d, V
done
clear
D)
p, T
done
clear
View Answer play_arrow
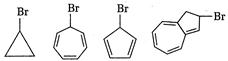
question_answer 94)
Which one of the following will quickly react with\[AgN{{O}_{3}}\]?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 95) The number of tetrahedral and octahedral void per unit cell of cubic closed packed structure is
A)
4, 8
done
clear
B)
4, 4
done
clear
C)
8, 4
done
clear
D)
8, 8
done
clear
View Answer play_arrow
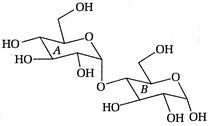
question_answer 96)
In the following disaccharide,
A)
Ring [A] is pyranonewith a-glycosidiclink
done
clear
B)
Ring [A] is furanone with a-glycosidic link
done
clear
C)
Ring [B] is pyranone with (3-glycosidic link
done
clear
D)
Ring [B] is furanone with a-glycosidic link
done
clear
View Answer play_arrow
question_answer 97) When a dilute solution of ammonia is saturated with\[{{H}_{2}}S\]it gives
A)
\[{{(N{{H}_{4}})}_{2}}S\]
done
clear
B)
\[N{{H}_{4}}HS\]
done
clear
C)
\[{{(N{{H}_{3}})}_{2}}{{H}_{2}}S\]
done
clear
D)
\[N{{H}_{3}}.{{H}_{2}}S\]
done
clear
View Answer play_arrow
question_answer 98) If\[E_{{{M}^{+}}/M}^{0}=-1.2V,\]\[E_{{{x}_{2}}/{{x}^{-}}}^{0}=-1.1V\]and\[E_{{{O}_{2}}/{{H}_{2}}O}^{0}=1.23V,\]then on electrolysis of aqueous solution of salt MX, the products obtained are
A)
\[M,{{X}_{2}}\]
done
clear
B)
\[{{H}_{2}},{{X}_{2}}\]
done
clear
C)
\[{{H}_{2}},{{O}_{2}}\]
done
clear
D)
\[M,{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 99) For the process to occur under adiabatic condition, the correct condition is
A)
\[\Delta T=0\]
done
clear
B)
\[\Delta U=0\]
done
clear
C)
\[\Delta p=0\]
done
clear
D)
\[\Delta q=0\]
done
clear
View Answer play_arrow
question_answer 100) Which of the element is available in carbonic anhydrase?
A)
\[Pd\]
done
clear
B)
\[Fe\]
done
clear
C)
\[Zn\]
done
clear
D)
\[Pt\]
done
clear
View Answer play_arrow
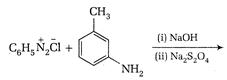
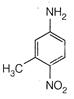
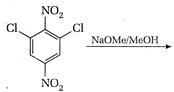
question_answer 101)
Identify reactant [A] for the following reaction
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 102)
Identify the product of the following reaction.
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 103)
The absolute configuration of the following compound is
A)
R, R, R
done
clear
B)
R, R, S
done
clear
C)
R, S, R
done
clear
D)
S, R, R
done
clear
View Answer play_arrow
question_answer 104) The wrong statement among the following is
A)
acid rain is mostly because of oxides of nitrogen and sulphur
done
clear
B)
green house effect is responsible for global warming
done
clear
C)
ozone layer does not permit infrared infrared radiation from the sun to reach earth
done
clear
D)
chlorofluorocarbons are responsible for ozone layer depletion
done
clear
View Answer play_arrow
question_answer 105) The vapour pressure of pure benzene at certain temperature is 1 bar. A non-volatile, non-electrolyte solid weighing 2 g when added to 39 g of benzene (molar mass\[78\text{ }g\text{ }mo{{l}^{-1}}\]) yields solution of vapour pressure of 0.8 bar. The molar mass of solid substance is
A)
32
done
clear
B)
16
done
clear
C)
64
done
clear
D)
48
done
clear
View Answer play_arrow
question_answer 106) \[HCI{{O}_{4}}.2{{H}_{2}}O\]after reaction with fuming sulphuric acid generates.
A)
\[Cl{{O}_{2}}+{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[C{{l}_{2}}{{O}_{7}}+{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
\[HCl{{O}_{4}}+{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[C{{l}_{2}}{{O}_{6}}+{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 107) The compound formed upon combustion of potassium metal in excess air is
A)
\[{{K}_{2}}{{O}_{2}}\]
done
clear
B)
\[K{{O}_{2}}\]
done
clear
C)
\[{{K}_{2}}O\]
done
clear
D)
\[KOH\]
done
clear
View Answer play_arrow
question_answer 108) Energy of activation of forward reaction for an endothermic process is 90 kJ. If enthalpy change for the reaction is 50 kJ then activation energy for backward reaction will be
A)
40kJ
done
clear
B)
140kJ
done
clear
C)
90 kJ
done
clear
D)
50 kJ
done
clear
View Answer play_arrow
question_answer 109) For ion\[O_{2}^{-}\]the bond order is
A)
2
done
clear
B)
1.5
done
clear
C)
2.5
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 110) Extraction of mercury from cinnabar is achieved by
A)
heating it in air
done
clear
B)
electrolytic reduction
done
clear
C)
roasting followed by reduction with caroon
done
clear
D)
roasting followed by reduction with another metal
done
clear
View Answer play_arrow
question_answer 111) The correct order of the ligands, \[O{{H}^{-}},NO_{3}^{-},PP{{h}_{3}},\]pyridine, according to their increasing field strength is
A)
\[NO_{3}^{-}<O{{H}^{-}}<pyridne<PP{{h}_{3}}\]
done
clear
B)
\[O{{H}^{-}}<NO_{3}^{-}<PP{{h}_{3}}<pyridne\]
done
clear
C)
\[O{{H}^{-}}<NO_{3}^{-}<pyridne<PP{{h}_{3}}\]
done
clear
D)
\[NO_{3}^{-}<O{{H}^{-}}<PP{{h}_{3}}<pridine\]
done
clear
View Answer play_arrow
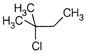
question_answer 112) When\[{{(C{{H}_{3}})}_{3}}CC{{H}_{2}}Cl\]is heated at\[300{}^\circ C,\]it gives
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 113) If the density of methanol is\[0.8\text{ }kg\text{ }{{L}^{-1}},\] what is its volume needed for making 4 L of its 0.25 M solution?
A)
4 mL
done
clear
B)
8 mL
done
clear
C)
40 mL
done
clear
D)
80 mL
done
clear
View Answer play_arrow
question_answer 114) The number of Na atom in 46 g Na (atomic weight of Na = 23) is
A)
\[6.023\times {{10}^{23}}\]
done
clear
B)
2
done
clear
C)
1
done
clear
D)
\[12.046\times {{10}^{23}}\]
done
clear
View Answer play_arrow
question_answer 115) If the solubility of a sparingly soluble salt\[A{{X}_{2}}\] is s mol/L, the solubility product is
A)
\[4{{s}^{3}}\]
done
clear
B)
\[8{{s}^{3}}\]
done
clear
C)
\[4{{s}^{2}}\]
done
clear
D)
\[{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 116) Which one of the following does not have\[s{{p}^{3}}\] hybridisation?
A)
\[C{{H}_{4}}\]
done
clear
B)
\[Xe{{F}_{4}}\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 117) Milk is an example of
A)
emulsion
done
clear
B)
sol
done
clear
C)
gel
done
clear
D)
foam
done
clear
View Answer play_arrow
question_answer 118) \[\frac{{{K}_{p}}}{{{K}_{C}}}\]for the reaction \[A(g)+2{{B}_{2}}(g)\xrightarrow{{}}A{{B}_{2}}(g)\]is
A)
\[RT\]
done
clear
B)
\[{{(RT)}^{2}}\]
done
clear
C)
\[\frac{1}{RT}\]
done
clear
D)
\[\frac{1}{{{(RT)}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 119) One mole of an ideal gas expands isothermally and reversibly from 2 L to 20 L at 300 K. If the final pressure of the gas is 1 bar, the work done by the gas is
A)
\[-300\,R\,in\,10\]
done
clear
B)
\[300\,R\,in\,10\]
done
clear
C)
\[18\]
done
clear
D)
\[-18\]
done
clear
View Answer play_arrow
question_answer 120) A unit cell with edge length\[a\ne b\ne c\]and axial angle\[\alpha =\beta =\gamma =90{}^\circ \]is called
A)
cubic
done
clear
B)
tetragonal
done
clear
C)
orthorhombic
done
clear
D)
hexagonal
done
clear
View Answer play_arrow
question_answer 121) Which of the following pairs in angiosperms are diploid and triploid, respectively?
A)
Polar nucleus and secondary nucleus
done
clear
B)
Microspore mother cell and egg cell
done
clear
C)
Secondary nucleus and endosperm
done
clear
D)
Endosperm and antipodal cells
done
clear
View Answer play_arrow
question_answer 122) What is the function of germ pore?
A)
Initiation of pollen tube
done
clear
B)
Absorption of water for seed germination
done
clear
C)
Emergence of radical
done
clear
D)
Release of male gametes
done
clear
View Answer play_arrow
question_answer 123) In\[{{C}_{3}}-\]plants, the first stable compound formed after\[C{{O}_{2}}\]fixation is
A)
oxaloacetic acid
done
clear
B)
malic acid
done
clear
C)
phosphoglyceraldehyde
done
clear
D)
3-phosphoglycerate
done
clear
View Answer play_arrow
question_answer 124) The part of Fallopian tube closest to the ovary is
A)
infundibulum
done
clear
B)
isthmus
done
clear
C)
ampulla
done
clear
D)
cervix
done
clear
View Answer play_arrow
question_answer 125) As secondary growth proceeds, in a dicot stem, the thickness of
A)
sapwood increases
done
clear
B)
heartwood increases
done
clear
C)
Both [a] and [b]
done
clear
D)
Both sapwood and heartwood remains the same
done
clear
View Answer play_arrow
question_answer 126) The tissue which covers the external surface of the animal body and the internal surface of visceral organs is
A)
epithelial tissues
done
clear
B)
connective tissue
done
clear
C)
adipose tissue
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 127) Amount of aie remains after deep breathing is called
A)
dead space
done
clear
B)
residual volume
done
clear
C)
vital capacity
done
clear
D)
ventilation rate
done
clear
View Answer play_arrow
question_answer 128) A transgenic food crop which may help in solving the problem of night-blindness in developing countries is
A)
Golden rice
done
clear
B)
Flavr savr tomatoes
done
clear
C)
starlink maize
done
clear
D)
Bt soybean
done
clear
View Answer play_arrow
question_answer 129) The basic functional unit of human kidney is
A)
Henles loop
done
clear
B)
nephron
done
clear
C)
nephridia
done
clear
D)
pyramid
done
clear
View Answer play_arrow
question_answer 130) What is the inner lining of the uterus called?
A)
Cervix
done
clear
B)
Oviduct
done
clear
C)
Endometrium
done
clear
D)
Fimbriae
done
clear
View Answer play_arrow
question_answer 131) Common character at all vertebrates without exception is
A)
body divided into head, trunk and tail
done
clear
B)
two pairs of limbs
done
clear
C)
exoskeleton
done
clear
D)
the presence of skull
done
clear
View Answer play_arrow
question_answer 132) Energy flow in ecosystem is
A)
bidirectional
done
clear
B)
multidirectional
done
clear
C)
unidirectional
done
clear
D)
all around
done
clear
View Answer play_arrow
question_answer 133) The polluting strength of sewage is usually characterised by its
A)
BOD
done
clear
B)
nitrogen content
done
clear
C)
ozone content
done
clear
D)
eutrophication
done
clear
View Answer play_arrow
question_answer 134) Most plants obtain their nitrogen from the soil in the form of
A)
nitric acid
done
clear
B)
free nitrogen gas
done
clear
C)
nitrates
done
clear
D)
nitrite
done
clear
View Answer play_arrow
question_answer 135) Coelom is lined on all sides by
A)
ectoderm
done
clear
B)
mesoderm
done
clear
C)
endoderm
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 136) Mating of an organism to a double recessive in order to determine wither it is homozygous or heterozygous for character under consideration is called
A)
reciprocal cross
done
clear
B)
test cross
done
clear
C)
dihybrid cross
done
clear
D)
back cross
done
clear
View Answer play_arrow
question_answer 137) During meiosis\[-I,\] the number of chromosomes is
A)
halved
done
clear
B)
tripled
done
clear
C)
doubted
done
clear
D)
quadrupled
done
clear
View Answer play_arrow
question_answer 138) Which one of the following is not a correct explanation of cross-pollination?
A)
The pollen grains of male flowers are transferred to the stigma of the female flowers.
done
clear
B)
The pollen grains are transferred from one flower to another flower, of another plant of the same species.
done
clear
C)
The pollen grains are transferred from one flower to another flower situated on the same plant.
done
clear
D)
The pollen grains of one flower are transferred to the stigma of the same flower.
done
clear
View Answer play_arrow
question_answer 139) DNA replication takes place during
A)
S-phase
done
clear
B)
\[{{G}_{2}}-\]phase
done
clear
C)
\[{{G}_{1}}-\]phase
done
clear
D)
prophase
done
clear
View Answer play_arrow
question_answer 140) A character which is expressed in a hybrid is called
A)
dominant
done
clear
B)
recessive
done
clear
C)
codominant
done
clear
D)
epistatic
done
clear
View Answer play_arrow
question_answer 141) World AIDS day is on
A)
May 1
done
clear
B)
December 1
done
clear
C)
December 20
done
clear
D)
June 1
done
clear
View Answer play_arrow
question_answer 142) Which one of the following is not a micronutrient for plants?
A)
Magnesium
done
clear
B)
Molybdenum
done
clear
C)
Boron
done
clear
D)
Zinc
done
clear
View Answer play_arrow
question_answer 143) Conditions of a karyotype\[2n+/-2\]and \[2n\text{ +}/-2\]are called
A)
aneuploidy
done
clear
B)
polypioidy
done
clear
C)
autopolyloidy
done
clear
D)
monosomy
done
clear
View Answer play_arrow
question_answer 144) The idea of Natural Selection as the fundamental process of evolutionary changes was reached
A)
independently by Charles Darwin and Alfred Russel Wallace in 1900
done
clear
B)
By diaries Darwin in 1866
done
clear
C)
By Alfred Russel Wallace in 1901
done
clear
D)
independently by Charles Darwin and Alfred Russel Wallace in 1859
done
clear
View Answer play_arrow
question_answer 145) Genes for cytoplasmic male sterility in plants are generally located in
A)
nuclear-genome
done
clear
B)
mitochondrial genome
done
clear
C)
chloroplast genome
done
clear
D)
cytosol
done
clear
View Answer play_arrow
question_answer 146) The first body segment of earthworm is
A)
peristome
done
clear
B)
peristomium
done
clear
C)
protostomium
done
clear
D)
protostome
done
clear
View Answer play_arrow
question_answer 147) Arrange the following in ascending order of Linnaean hierarchy.
A)
Kingdom - Phylum - Class - Order - Family - Genus - Species
done
clear
B)
Kingdom - Family - Genus - Species - Class - Phylum - Order
done
clear
C)
Kingdom - Order - Species - Genus - Class - Family - Phylum
done
clear
D)
Species - Genus - Family - Order - Class - Phylum-Kingdom
done
clear
View Answer play_arrow
question_answer 148) Concentration of the urine is controlled by
A)
MSH
done
clear
B)
ADH
done
clear
C)
Oxytocin
done
clear
D)
ACTH
done
clear
View Answer play_arrow
question_answer 149) Double fertilisation involves
A)
fertilisation of the egg by two male gametes
done
clear
B)
fertilisation of two eggs in the same embryo sac by two sperms brought by one pollen tube
done
clear
C)
fertilisation of the egg and the central cell by two sperms brought by different pollen tubes
done
clear
D)
fertilisation of the egg and the central cell by two sperms brought by the same pollen tube
done
clear
View Answer play_arrow
question_answer 150) Yeast is used in the production of
A)
bread and beer
done
clear
B)
cheese and butter
done
clear
C)
citric acid and lactic acid
done
clear
D)
lipase and pectinase
done
clear
View Answer play_arrow
question_answer 151) Which one of the following statements is/are correct?
A)
FSH and LH occur in both males and females
done
clear
B)
FSH and LH stimulate the follicle to secrete oestrogen
done
clear
C)
The ovarian cycle depends on the blood. levels of FSH and LH
done
clear
D)
All of these are correct
done
clear
View Answer play_arrow
question_answer 152) The letter T in T-lymphocyte refers to
A)
thymus
done
clear
B)
thyroid
done
clear
C)
tonsil
done
clear
D)
thalamus
done
clear
View Answer play_arrow
question_answer 153) Plant species having a wide range of genetical distribution evolve into a local population known as
A)
ecotype
done
clear
B)
biome
done
clear
C)
ecosystem
done
clear
D)
population
done
clear
View Answer play_arrow
question_answer 154) Bacteria cannot survive in a highly salted pickle because
A)
salt inhibits reproduction of bacteria
done
clear
B)
enough light is available for photosynthesis
done
clear
C)
they become plasmolysed and death occurs
done
clear
D)
nutrients in the pickle medium cannot support life
done
clear
View Answer play_arrow
question_answer 155) The blood does not clot inside the body because of
A)
oxygenation of blood
done
clear
B)
movement of blood
done
clear
C)
presence of heparin in blood
done
clear
D)
absence of fibrinogen in blood
done
clear
View Answer play_arrow
question_answer 156) Which one of the following is the most abundant protein in the animal world?
A)
Collagen
done
clear
B)
Insulin
done
clear
C)
Trypsin
done
clear
D)
Haemoglobin
done
clear
View Answer play_arrow
question_answer 157) The function of leghaemoglobin in the root nodules of legumes is
A)
oxygen removal
done
clear
B)
inhibition of nitrogenase activity
done
clear
C)
expression of nif gene
done
clear
D)
nodule differentiation
done
clear
View Answer play_arrow
question_answer 158) Which of the following is a bacterium involved in denitrification?
A)
Azotobacter
done
clear
B)
Nitrosomonas
done
clear
C)
PsQudomonas
done
clear
D)
Nitrobacter
done
clear
View Answer play_arrow
question_answer 159) There are .......... pairs of cranial nerves arising from the brain of human beings.
A)
8
done
clear
B)
12
done
clear
C)
18
done
clear
D)
25
done
clear
View Answer play_arrow
question_answer 160) Which one of the following membranes secretes a watery fluid that lubricates and cushions the joint?
A)
Tendons
done
clear
B)
Ligaments
done
clear
C)
Cartilage
done
clear
D)
Synovial membrane
done
clear
View Answer play_arrow
question_answer 161) Which one of the following causes the mammary glands to enlarge at puberty?
A)
Testosterone
done
clear
B)
Progesterone
done
clear
C)
Oestrogen
done
clear
D)
Oxytocip
done
clear
View Answer play_arrow
question_answer 162) Phenotype of an organism is the result of
A)
environmental changes and sexual dimorphism
done
clear
B)
cytoplasmic effects and nutrition
done
clear
C)
mutations and linkages
done
clear
D)
genotype and environment interactions
done
clear
View Answer play_arrow
question_answer 163) The Ley dig cells found in the human body are the secretory source of
A)
glucagon
done
clear
B)
androgens
done
clear
C)
progesterone
done
clear
D)
intestinal mucus
done
clear
View Answer play_arrow
question_answer 164) Which one of the following statements is not true with respect to viability of mammalian sperm?
A)
Viability of sperm is determined by its motility.
done
clear
B)
Sperms must be concentrated in a thick suspension.
done
clear
C)
Sperm is viable for only up to 24 hours.
done
clear
D)
Survival of the sperm depends on the pH of the medium and it is most active in alkaline pH.
done
clear
View Answer play_arrow
question_answer 165) In a lake phytoplankton grow in abundance in
A)
littoral zone
done
clear
B)
limnetic zone
done
clear
C)
profundal zone
done
clear
D)
benthic region
done
clear
View Answer play_arrow
question_answer 166) Examples of secondary air pollutants is /are
A)
Smog
done
clear
B)
\[{{O}_{3}}\]
done
clear
C)
PAN
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 167) Evolution of different species in a given area starting from a point and spreading to other geographical areas is known as
A)
migration
done
clear
B)
divergent evolution
done
clear
C)
adaptive radiation
done
clear
D)
natural section
done
clear
View Answer play_arrow
question_answer 168) The monocotyledonous seed consists of one large and shield shaped cotyledon known as a/an
A)
coleoptile
done
clear
B)
scutellum
done
clear
C)
aleurone layer
done
clear
D)
coleorhiza
done
clear
View Answer play_arrow
question_answer 169) Closed vascular bundles lack
A)
cambium
done
clear
B)
pith
done
clear
C)
ground tissue
done
clear
D)
conjunctive tissue
done
clear
View Answer play_arrow
question_answer 170) Restriction endonucleases
A)
are synthesised by bacteria as part of their defense mechanism
done
clear
B)
are used for in vitro DNA synthesis
done
clear
C)
are used in genetic engineering for ligation of two DNA molecules
done
clear
D)
are present in mammalian cells for degradation of DNA when the cell dies
done
clear
View Answer play_arrow
question_answer 171) In five kingdom classification single celled eukaryotes are included in
A)
Fungi
done
clear
B)
Protista
done
clear
C)
Monera
done
clear
D)
Archaea
done
clear
View Answer play_arrow
question_answer 172) Emulsification of fat occurs by
A)
bile salts
done
clear
B)
bile pigments
done
clear
C)
pancreatic juice
done
clear
D)
succus entericus
done
clear
View Answer play_arrow
question_answer 173) In Mendels experiments with garden pea, round seed shape (RR) was dominant over wrikled seed (rr), yellow cotyledon (YY) was dominant over green cotyledon (yy). What are the expected phenotypes in the \[{{F}_{2}}-\]generation of the cross \[RRYY\times rryy\]?
A)
Only wrinkled seeds with green cotyledons
done
clear
B)
Only wrinkled seeds with yellow cotyledons
done
clear
C)
Only round seeds with green cotyledons
done
clear
D)
Round seeds with yellow cotyledons, round seeds with green cotyledons, wrinkled seeds with yellow cotyledons and wrinkled seeds with green cotyledons
done
clear
View Answer play_arrow
question_answer 174) The biological control of agricultural pests unlike chemical control is
A)
very expensive
done
clear
B)
polluting
done
clear
C)
self perpetuating
done
clear
D)
toxic
done
clear
View Answer play_arrow
question_answer 175) AIDS is caused by
A)
blood cancer
done
clear
B)
TMV
done
clear
C)
bacterium
done
clear
D)
human immunodeficiency virus
done
clear
View Answer play_arrow
question_answer 176) Which type of white blood cells are concerned with the release of histamine and the natural anticoagulant heparin?
A)
Monocytes
done
clear
B)
Neutrophils
done
clear
C)
Basophils
done
clear
D)
Eosinophils
done
clear
View Answer play_arrow
question_answer 177) The catalytic efficiency of two different enzymes can be compared by the
A)
molecular size of the enzymes
done
clear
B)
pH optimum values
done
clear
C)
Km values
done
clear
D)
formation of the product
done
clear
View Answer play_arrow
question_answer 178) The enzyme responsible for primary carboxylation in\[{{C}_{3}}-\]plants is
A)
pyruvate carboxylase
done
clear
B)
succinic dehydrogenase
done
clear
C)
hexokinase
done
clear
D)
RuBP carboxylase /oxygenase
done
clear
View Answer play_arrow
question_answer 179) Which of the following enzymes is used to join DNA fragments?
A)
DNA polymerase
done
clear
B)
Ligase
done
clear
C)
Prirnase
done
clear
D)
Endonuclease
done
clear
View Answer play_arrow
question_answer 180) The word species was coined by
A)
Aristotle
done
clear
B)
Linnaeus
done
clear
C)
John Ray
done
clear
D)
Engler
done
clear
View Answer play_arrow