A) 840 K
B) 280 K
C) 560 K
D) 380 K
Correct Answer: D
Solution :
Carnot engine is a reversible device which draws heat from a hotter reservoir at temperature\[{{T}_{1}}\], produces a positive amount of work in the surroundings and discharges rest amount of heat into a colder reservoir at temperature\[{{T}_{2}}\].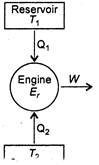 Efficiency is defined as \[\eta =1-\frac{{{T}_{2}}}{{{T}_{1}}}\] \[\frac{50}{100}=1-\frac{273+7}{{{T}_{1}}}\] \[\Rightarrow \] \[\frac{1}{2}=1-\frac{280}{{{T}_{1}}}\] \[\Rightarrow \] \[\frac{280}{{{T}_{1}}}=\frac{1}{2}\] \[\Rightarrow \] \[{{T}_{1}}=560\,K\] Let new temperature of high temperature reservoir is\[T_{1}^{}\]. Then, \[\frac{70}{100}=1-\frac{280}{T_{1}^{}}\] \[\Rightarrow \] \[\frac{280}{T_{1}^{}}=\frac{3}{10}\] \[\Rightarrow \] \[T_{1}^{}=\frac{280\times 10}{3}=933\,K\] \[\therefore \]Increase in temperature \[=933-560=373K-380K\]
Efficiency is defined as \[\eta =1-\frac{{{T}_{2}}}{{{T}_{1}}}\] \[\frac{50}{100}=1-\frac{273+7}{{{T}_{1}}}\] \[\Rightarrow \] \[\frac{1}{2}=1-\frac{280}{{{T}_{1}}}\] \[\Rightarrow \] \[\frac{280}{{{T}_{1}}}=\frac{1}{2}\] \[\Rightarrow \] \[{{T}_{1}}=560\,K\] Let new temperature of high temperature reservoir is\[T_{1}^{}\]. Then, \[\frac{70}{100}=1-\frac{280}{T_{1}^{}}\] \[\Rightarrow \] \[\frac{280}{T_{1}^{}}=\frac{3}{10}\] \[\Rightarrow \] \[T_{1}^{}=\frac{280\times 10}{3}=933\,K\] \[\therefore \]Increase in temperature \[=933-560=373K-380K\]
You need to login to perform this action.
You will be redirected in
3 sec
