A)
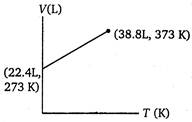
B)
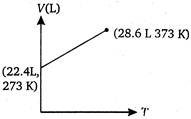
C)
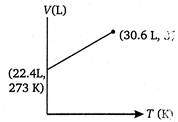
D)
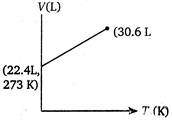
Correct Answer: C
Solution :
Volume of 1 mole of an ideal gas at 273 K and 1 atm pressure = 22.4 L \[\therefore \]volume at 373 K and 1 atm pressure will be given by \[\frac{{{V}_{1}}}{{{T}_{1}}}=\frac{{{V}_{2}}}{{{T}_{2}}}\](at constant p) \[={{V}_{2}}=30.6L\] \[=\frac{22.4}{273}=\frac{V}{373}\] \[{{V}_{2}}=30.6L\]You need to login to perform this action.
You will be redirected in
3 sec
