A) \[{{C}_{6}}{{H}_{5}}OH>{{C}_{6}}{{H}_{5}}COOH>C{{H}_{3}}COOH\]
B) \[{{C}_{6}}{{H}_{5}}COOH>C{{H}_{3}}COOH>{{C}_{6}}{{H}_{5}}OH\]
C) \[C{{H}_{3}}COOH>{{C}_{6}}{{H}_{5}}COOH>{{C}_{6}}{{H}_{5}}OH\]
D) \[{{C}_{6}}{{H}_{5}}OH>C{{H}_{3}}COOH>{{C}_{6}}{{H}_{5}}COOH\]
Correct Answer: B
Solution :
\[{{C}_{6}}{{H}_{5}}\]group is electron withdrawing group, hence it is the strongest carboxylic acid. \[C{{H}_{3}}COOH\]is more acidic than phenol because of more resonance stabilisation of the \[C{{H}_{3}}CO{{O}^{-}}\]ion due to the presence of equivalent resonating structures than the phenoxide ion. \[C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ |\,| \end{smallmatrix}}{\mathop{C}}\,-O\underset{{}}{\overset{{}}{\longleftrightarrow}}C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ |\, \end{smallmatrix}}{\mathop{C}}\,=O\] equivalent resonating structures of acetate ion. Non-equivalent resonating structure of phenoxide ion.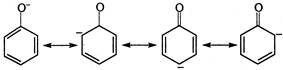 So, the correct order is\[{{C}_{6}}{{H}_{5}}COOH>C{{H}_{3}}COOH>{{C}_{6}}{{H}_{5}}OH\].
So, the correct order is\[{{C}_{6}}{{H}_{5}}COOH>C{{H}_{3}}COOH>{{C}_{6}}{{H}_{5}}OH\].
You need to login to perform this action.
You will be redirected in
3 sec
