A) 2,2-dimethyl-l-butene
B) 2,3-dimethyl-l-butene
C) 2,3-dimethyl-2-butene
D) \[cis\]and trans isomers of 2,3-dimethyl-2- butane
Correct Answer: C
Solution :
\[{{H}_{3}}C-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}+cone\text{ }{{H}_{2}}S{{O}_{4}}\] \[\xrightarrow[{}]{\Delta }\underset{(minor)}{\mathop{{{H}_{3}}C-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-CH=C{{H}_{2}}}}\,+\underset{(major)}{\mathop{C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,=\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}}}\,\] Mechanism
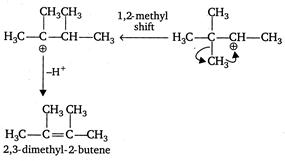 Note Most stable alkene is the major product of the reaction. C is and trans isomerism is not possible because similar groups are present on both double bonded carbon atoms.
Note Most stable alkene is the major product of the reaction. C is and trans isomerism is not possible because similar groups are present on both double bonded carbon atoms.
You need to login to perform this action.
You will be redirected in
3 sec
