A) \[112\]
B) \[512\]
C) \[400\]
D) \[614\]
Correct Answer: B
Solution :
Key Idea: The rate of reaction increases by \[{{(2)}^{n}}\]for every \[10{}^\circ \] rise in temperature.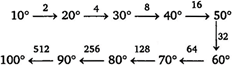 \[\therefore \]rate of reaction increase \[512\] times.
\[\therefore \]rate of reaction increase \[512\] times.
You need to login to perform this action.
You will be redirected in
3 sec
